
City of Santa Barbara
Public Works Department
Memorandum
DATE: February 17, 2022
TO: Water Commission
VIA: Joshua Haggmark, Water Resources Manager
FROM: Arturo Keller, Water Commissioner
SUBJECT: What Is Direct Potable Reuse And What Are Some Key Considerations
For Its Implementation In California
The State Water Resource Control Board has been actively working on developing
regulations that would support state wide Direct Potable Reuse (DPR). Is California ready
for DPR? In this presentation, we will explore the technological, economic, and
environmental considerations associated with DPR.
ATTACHMENT: Direct Potable Reuse: Are We Ready? A Review of Technological,
Economic, and Environmental Considerations
6-b
Direct Potable Reuse: Are We Ready? A Review of Technological,
Economic, and Environmental Considerations
Arturo A. Keller,* Yiming Su, and David Jassby
Cite This: https://doi.org/10.1021/acsestengg.1c00258
Read Online
ACCESS
Metrics & More Article Recommendations
*
sı
Supporting Information
ABSTRACT: Meeting future water demand will require serious consideration of direct potable reuse
(DPR) for many water agencies. There has been tremendous progress in the technologies needed to
address the concerns that conventional and novel water contaminants pose. Yet, to date, only a few
relatively small DPR operations have been installed. As we get closer to the point where regulations are
finalized and serious investments are planned, there is a need to ask: Are we ready? In this Review, we
explore the technological, economic, and environmental considerations associated with DPR. In
particular, we focus on the status of technologies for addressing the most challenging water pollutants,
the cost estimates for DPR, and the energy demand and associated implications of DPR. We find that,
although the technologies are nearly ready for DPR, the most critical issue will be real-time monitoring
of a number of molecules that pose distinct challenges to advanced treatment trains. In addition, there
is a need to consider emergency planning, both in terms of emergency buffer reservoir(s) and planning
for situations in which the treated water does not meet specifications. Since any advanced treatment
train will result in a significant increase in embedded energy, it will be particularly important to plan for
renewable energy to minimize environmental impacts.
KEYWORDS: Emerging pollutants, water quality, reuse, monitoring, sensors
■
INTRODUCTION
Rising temperatures, increasing variability in precipitation
patterns, more extended droughts, growing populations, and
limited alternatives for new traditional water sources in semiarid
regions with frequent water scarcity episodes, such as the
southwestern US and around the world, are major drivers for a
serious consideration of direct potable reuse (DPR).
1
The
concept of “closing the loop”
2
in terms of the urban water cycle,
as part of the “Fourth Water Revolution”, considers potable
water reuse as one of its key pillars.
3
The employment of high-
quality treated wastewater for planned indirect potable reuse
(IPR), by employing an environmental buffer as in the case of
groundwater recharge or reservoir water augmentation, is
already a current practice in many regions (e.g., NEWater
Singapore,
4,5
Orange County Water District Groundwate r
Replenishment System (California),
6
Upper Occoquan Service
Authority (Virginia),
7
Montebello Forebay Groundwater
Recharge Project (Los Angeles, California), Western Corridor
Recycled Water Scheme (South East Queensland, Australia)
8
).
Summaries of several pilot or operational DPR and IPR systems
are provided by Guo et al.
9
However, the direct introduction of
high-quality treated effluent to a public water system or for raw
water augmentation immediately upstream of a water treatment
plant, as planned in DPR, requires additional considera-
tions.
10−12
Given the high cost of treating water for potable reuse, the first
step must be to implement a community-wide water
conservation plan. There are many success stories, throughout
California, the US, and around the world,
13−20
that implement
appliances and fixtures with higher water (and energy) use
efficiency, convert landscapes to vegetation that requires almost
no watering or t o permeabl e hardscapes, eliminate leaks
throughout the entire water system, and provide incentives to
consumers.
21,22
The employment of an environmental buffer, essentially
storing the treated water in a large compartment, either an
aquifer or a reservoir, provides a dilution of constituents that
may be present in the treated water as well as time for natural
attenuation and detection of any unexpected changes in water
quality.
23−26
In California, Title 22 requires that water for IPR
be treated with reverse osmosis (RO) and an advanced oxidation
process (AOP) plus a minimum of two months of subsurface
travel time or reservoir retention time with specific consid-
erations for dilution ratios.
27
In fact, a longer residence time,
greater than 6 months, is desirable; less than two months
requires additional considerations. The goal is to ensure that the
concentrations of inorganic and organic chemicals are below
their respective maximum concentration levels (MCLs), water
quality objectives (WQOs), or notification levels (NLs), that
Special Issue: Technology Baselines and Innovation
Priorities for Water Treatment and Supply
Received: July 8, 2021
Revised: September 16, 2021
Accepted: September 17, 2021
Reviewpubs.acs.org/estengg
© XXXX American Chemical Society
A
https://doi.org/10.1021/acsestengg.1c00258
ACS EST Engg. XXXX, XXX, XXX−XXX
Downloaded via UNIV OF CALIFORNIA SANTA BARBARA on September 30, 2021 at 21:59:22 (UTC).
See https://pubs.acs.org/sharingguidelines for options on how to legitimately share published articles.
ATTACHMENT

levels of enteric viruses have a 12-log reduction, and that levels of
Giardia cysts and Cryptos poridium oocysts have a 10-log
reduction.
27
However, many communities do not have a nearby
reservoir or aquifer capacity for the 2+ months of residence time
or dilution ratios, or the hydrogeochemistry may be unsuitable
for IPR. In addition, the elimination of the environmental buffer
may be more cost-efficient than IPR.
28
Many communities that extract their raw water from rivers,
such as the Ohio River, the Colorado River, and many others
around the world, already conduct unplanned IPR.
29,30
Upstream communities discharge their treated wastewater into
the river, in many cases with only secondary treatment, which is
diluted and then extracted downstream by other communities,
with much less than the recommended two months of residence
time. Technologies, regulations, and management approaches
that are relevant for DPR are thus valid for many other locations.
The elimination of the environmental buffer raises concerns
with regards to illegal or accidental discharges of chemicals into
the wastewater sewer collection stream or a stormwater
conveyance that connects to the wastewater system that may
not be regularly monitored.
31,32
Concerns about water security
also increase when there is a short time to react to such events.
The current COVID-19 pandemic has been a wake-up call to
also address unexpected, novel pathogens that may be present in
wastewater.
33−42
Thus, even for DPR, a small emergency buffer
should be built into the design to handle any flows that are
suspected of not meeting drinking water quality goals and a
means to divert flow temporarily to a receiving water body until
goals are met.
24,43,44
Closing the loop raises concerns with regards to the
accumulation of common constituents (e.g., chloride, nitrate,
borate) throughout the urban water system, even in the case of
IPR, where the constituents can increase in the aquifer or
reservoir. Current treatment levels for most wastewater
treatment plants (WWTPs) do not entirely remove chemicals
of emerging concern (CECs) such as pharmaceuticals and
personal care products (PPCPs), per- and polyfluoroalkyl
substances (PFASs) , disinfection byproducts (DBPs), and
nanomaterials and their residual ions as well as many industrial
chemicalsthatarenotregulated or monitored (e.g.,
acetone).
6,45−50
Conventional WWTPs are not required to
remove these contaminants, and although many can be partially
removed from the effluent, residuals can remain. The potential
effects of chronic exposure to low-level residuals of these
contaminants have not been fully characterized.
51−56
Thus,
there is a need for advanced treatment and real-time monitoring
of low (ng/L) levels of these currently unregulated contami-
nants. There is particular concern with low molecular mass
(<200 Da) neutral chemicals that may not be removed entirely
by RO and/or AOPs,
6
such as those presented in Table 1.
To address these concerns, advanced online monitoring of
chemicals and rapid off-line analytical capabilities will be
necessary.
57−63
Online monitoring of unregulated CECs cannot
rely on currently available total organic carbon (TOC) sensors,
even if they can detect organic molecules at 0.5 mg/L, or even
0.1 mg/L, since that is still orders of magnitude greater than the
ng/L levels at which the CECs may be present. Although
nontargeted and semitargeted analysis can be employed, these
methods are capital intensive and have high labor costs,
64
requiring novel tools for real-time monitoring. In the long term,
online monitoring will have to be reliable, convenient, and
affordable for WWTPs. Several sampling points will be needed
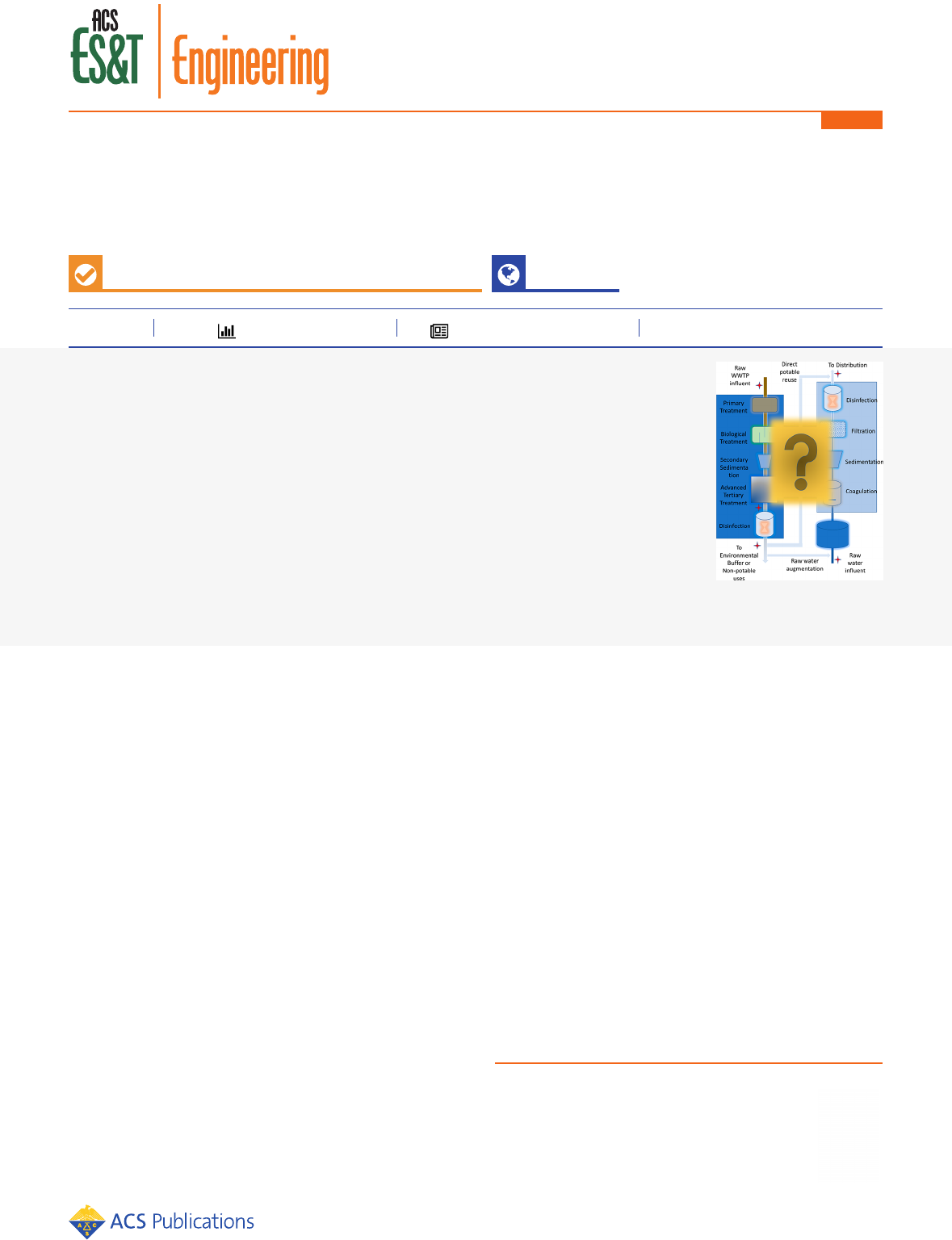
along the treatment trains and distribution systems (Figure 1).
In addition, raw wastewater contains a high level of pathogens,
including enteric bacteria, viruses, and protozoa. Raw waste-
water may contain virus concentrations of 107 to 109 gene
copies per liter for qPCR-based analyses, virus concentrations of
3 to 1300 per liter for culture-based analyses, and protozoa
concentrations of 6 to 17 000 per liter.
104
Their mass loading
significantly decreases through the conventional WWTP
process, and most can be removed by advanced technologies
such as RO and AOPs; however, there is a need to be vigilant and
implement online monitoring to avoid the risk of exposing the
population to these pathogens.
105,106
Sewersheds that include a significant number of industrial and
commercial activity will need additional administrative meas-
ures, such as identification of all possible sources, inventories of
chemicals in use, regular updating of inventories, enforcement of
Table 1. Partial List of Chemicals of Potential Concern after
Advanced Treatment
chemical potential effects reference
Inorganics
arsenic (arsenite) cancer and other diseases 65
boron developmental effects, toxic for plants 66
bromate carcinogen, developmental
neurotoxicity, negative effects on
crop plants
67, 68
chlorate carcinogen 69−71
Organics
1,4-dioxane liver and kidney damage 72
2,4,6-trichlorophenol carcinogen 71
2,4,6-trichloroanisole organoleptic threshold 73, 74
2,4-dichloroanisole organoleptic threshold 73, 74
2-methyl-isobomeol organoleptic threshold 73, 74
acetaldehyde carcinogen 71
acetone and other VOCs taste and odor 75
atenolol developmental toxicity 76, 77
benzoquinones DNA and protein damage 78
bisphenol A endocrine disruption 79
bromoacetonitrile carcinogen 71
bromoform carcinogens 80, 81
carbamazepine toxic to pregnant women and fetuses 82−84
chloroacetonitrile carcinogen 71, 80, 81
chloroform carcinogens 80, 81
dichloromethane carcinogen 71
enedials damage to hepatic proteins 85, 86
estrogen endocrine disruption 87
fipronil liver toxicity 88, 89
formaldehyde carcinogen 71
geosmin organoleptic threshold 73, 74
glyoxal oxidative stress 90, 91
halogenated disinfection
byproducts
carcinogens, mutagens 92
imidacloprid reproductive toxicity 88,
93
nitrosamines carcinogens 71, 92,
94, 95
N-
nitrosodimethylamine
(NDMA)
hepatotoxic and carcinogen 96
oxoenals damage to hepatic proteins 85, 86
perfluoroalkyl
substances (PFASs)
thyroid disorders, cancer 97, 98
tri(2-chloroethyl)
phosphate (TCEP)
carcinogen 76,
99−101
tris(1-chloro-2-propyl)
phosphate (TCPP)
DNA-damage potential 102
triclosan endocrine disruption 103
ACS ES&T Engineering pubs.acs.org/estengg Review
https://doi.org/10.1021/acsestengg.1c00258
ACS EST Engg. XXXX, XXX, XXX−XXX
B
pretreatment (i.e., treatment at the industrial or commercial
source), and education of all employees as to the potential
effects of any discharge into the sewer that could result in
unexpected chemicals passing through the treatment systems
and into the water distribution system.
Other important considerations for DPR include (1) very low
total dissolved solids (TDS) after RO, which requires proper
dosing of dissolved solids to avoid corrosion problems that can
result in distribution system failures (e.g., leaking old
distribution pipes) and possible leaching of Pb and Cu;
107−109
(2) disposal of the RO brine, which can be a challenge for
communities far from the ocean and with sensitive nearby
habitats;
110,111
(3) higher embedded energy by incorporating
RO, AOP, chlorination, and any additional processes after the
conventional WWTP and the corresponding impacts on
greenhouse gases and other issues;
11
(4) additional pumping
costs to transport water upgradient to the high points of the
watersheds;
112
(5) increased capital and operating costs; (6)
additional complexity and training within the treatment plants;
(7) redundant capital investment in key processes (e.g., RO, NF,
activated carbon) to ensure these treatment steps are 100%
operational; (8) the need for close cooperation and coordina-
tion between the WWTP(s) and the agencies in charge of raw
water treatment and distribution to maintain uniform flows,
manage emergency reservoir(s), and implement action plans in
case the treated effluent does not meet water quality objectives.
In this Review, we consider the: (1) technologies available for
advanced tertiary treatment applicable for DPR and their ability
to address the contaminants indicated in Table 1; (2) tools for a
cost-effectiveness comparison; (3) energy and other consid-
erations for advanced tertiary treatment trains; (4) the critical
role for sensors that are being proposed to meet the challenges of
real-time monitoring at multiple locations for chemicals of
potential concern as well as for pathogens; (5) final
recommendations on our readiness to deploy DPR.
■
TREATMENT TECHNOLOGIES: EFFECTIVENESS
AND CHALLENGES
Low-pressure microfiltration (MF) and ultrafiltration (UF)
membranes remove a significant fraction of particulates and
large (>200 Da) organic molecules remaining after conventional
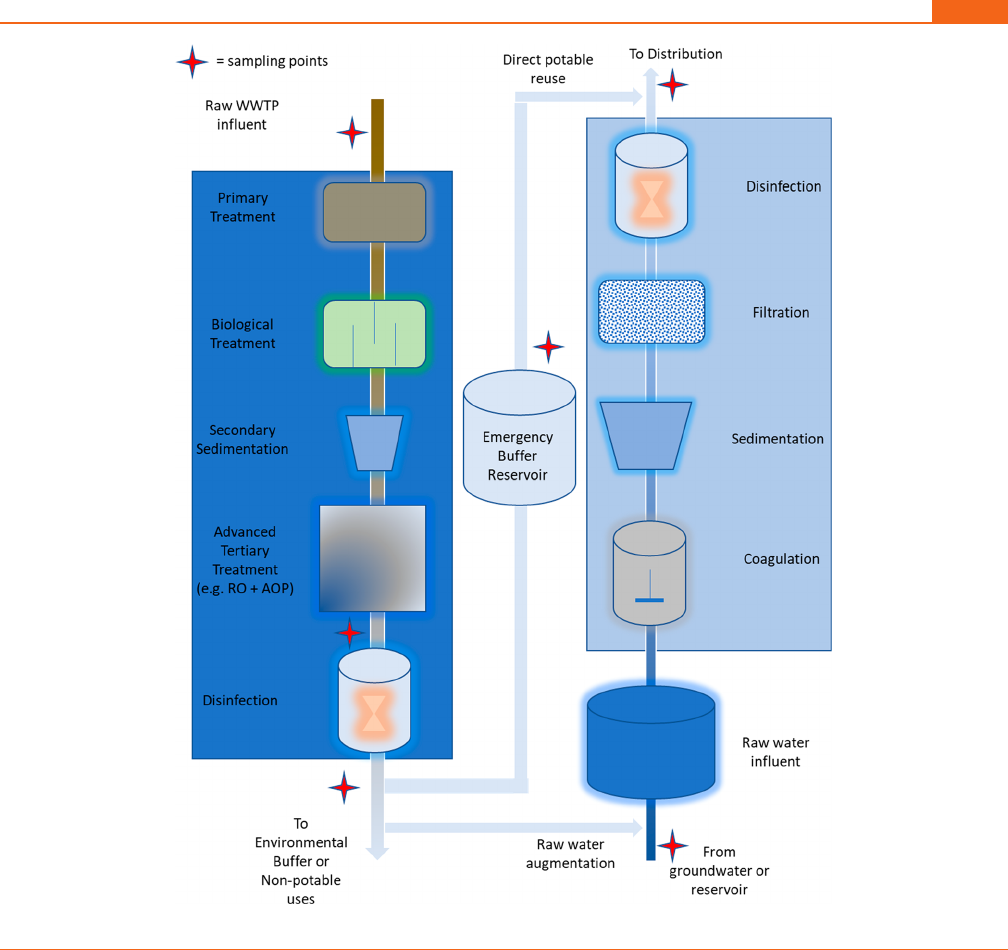
(primary and secondary) wastewater treatment (Figure 2).
Figure 1. Process diagram for direct and indirect potable reuse with potential sampling locations.
ACS ES&T Engineering pubs.acs.org/estengg Review
https://doi.org/10.1021/acsestengg.1c00258
ACS EST Engg. XXXX, XXX, XXX−XXX
C
Nanofiltration (NF) membranes can remove many additional
small molecules but do not remove low molecular mass
monovalent ions and cannot alone meet TDS requirements
for DPR. Reverse osmosis (RO) membranes remove most of the
remaining organic molecules (down to 150−200 Da) and
divalent ions as well as a fraction of the monovalent ions,
meeting TDS requirements. More than 90% of semivolatiles and
the majority of PPCPs and PFAS are well rejected by NF and
RO.
113−115
However, a significant fraction of small molecules,
such as chloroform, bromoform, bromodichloromethane, and
dibromochloromethane, are poorly rejected.
116
Short-chain
PFAS, TCEP, and other phosphoric acid esters can also pass
to the permeate at detectable concentrations.
76,117
A major
challenge for membrane technologies, in particular for RO and
NF membranes, is the irreversible adsorption of natural organic
matter and the formation of biofilms, which obstruct the passage
of water, known as fouling.
118−120
Particulate deposition can
also result in fouling.
121
Deposits of inorganic ions (i.e., scaling)
can also affect membrane performance over time, increasing the
pressure needed to maintain water flux.
121
Considerable effort
has been spent on reducing fouling and scaling via membrane
design, chemical additions, and pretreatment.
122,123
Biofilm
formation (biofouling) can be limited using disinfectants (e.g.,
chloramine), scaling by lowering pH and with antiscalants, and
NOM and particle deposition via UF and MF pretreat-
ment.
123,124
Significant advances have been made in membrane design and
performance using simulation tools from the molecular level to
system wide optimization. Molecular dynamics simulation of
membranes can serve to evaluate different membrane materials
in terms of treatment performance, energy requirements, and
fouling. In these studies, there is generally a focus on inorganic
ion (e.g., Na
+
,Cl
−
) transport through different membrane
materials. Molecular dynamics can serve to guide membrane
material development (e.g., use of functionalized carbon
nanotubes,
125
graphene,
126
and boron nitride nanotubes
127,128
for higher ion rejection and water flow rates), selectivity based
on membrane characteristics (e.g., pore size, membrane
structure and thickness, surface modifications), water and ion
dynamics, and fouling.
120,129
Membrane performance simu-
lations at larger scales with specific input water conditions (e.g.,
salinity, operating pressure and temperature, flow rate) can be
used to select among the many types of membranes available for
water reuse.
130
Simulations are also used to optimize system
design, consider single- vs two-step configurations, and
incorporate energy recovery devices and pretreatment as a
means to reduce energy requirements as well as other costs.
131
Process optimization can also be achieved using adaptive control
strategies for backwash frequency, disinfectant addition, RO
flux, water recovery (i.e., fraction of feedwater present in treated
water), pH, and antiscalants dose.
132
In addition to lowering
energy and chemical use, optimization can also increase
membrane lifetime and important operating costs.
132
AOPs can generally be divided into O
3
-based, UV-based,
plasma-based, and electrochemical approaches and can remove
many of the remaining organic molecules that pass through the
RO membranes, albeit with different effectiveness. For AOPs,
there have been a number of studies implementing artificial
neural networks (ANN) for determining removal efficiencies,
operational control, and optimization
133
as well as for predicting
performance.
134
In terms of removal efficiencies, the approach
to date is to consider specific target chemicals (e.g., textile
dyes,
135−138
pharmaceuticals,
139
pesticides, MTBE
140
) gener-
ally using a specific AOP,
141
but there is a need for a more
comprehensive approach. Overall, there is a major research gap
in the development of tools for predicting cost and performance
of AOPs in part due to the wide range of chemicals considered,
processes, and operational conditions.
A low-energy alternative to AOPs is granular activated carbon
(GAC), which can also be biologically enhanced (BAC) to
increase removal efficiency. For BAC, models have been
developed to predict the adsorption and biodegradation
performance as a function of operational conditions with good
accuracy.
142,143
Modeling was more accurate for poorly to
moderately adsorbing trace organics, indicating that the model is
more accurate for biodegradation if the kinetics are known.
143
A
few studies have modeled the combination of ozone and BAC to
predict the removal efficiency of trace organics in water reuse.
144
However, there is also a need for performance and optimization
models as well as to more accurately predict costs and energy
requirements based on operating conditions.
The focus of this section is on those molecules that pose
significant challenges for DPR, such as small monovalent ions
and organics.
Small Inorganic Molecules. The majority of heavy metals
(e.g., copper, chromium, nickel, etc.) are found in an ionic form
at neutral pH, are well-rejected by RO membranes, and are not
anticipated to be an issue in most streams.
146
However, As(III)
and boron, which are found as uncharged oxides/hydroxides at
neutral pH, are not well-rejected by RO membranes and could
pose a danger (although boron is a problem primarily for plants
and not mammals).
147−150
Arsenic is naturally present in many minerals, and although
typically dissolved concentrations are low, it can be found at up
to 2000 μg/L in some groundwater sources. While many water
supply systems in the US and around the world remove a
significant fraction of the arsenic load from their raw water, it
could be introduced into the system by domestic or industrial
users using local wells; careful monitoring is needed to avoid
buildup of As concentrations in a DPR system. Even at low
levels, arsenic can lead to a number of cancers (skin, lungs,
bladder, liver, kidney), and the effects may not be observed for
years until they are irreversible.
151
Although a fraction of the
particulate and dissolved arsenic can be removed by coagulation
or adsorption, it may not be sufficient. MF/UF have pore sizes
Figure 2. Size and molecular mass of pollutants and pathogens removed
by different techniques. DBPs = disinfection byproducts, EDCs =
endocrine disrupting chemicals, PPCPs = pharmaceuticals and personal
care products, and PFASs = per- and polyfluoroalkyl substances.
Membrane cutoff ranges from ref 145.
ACS ES&T Engineering pubs.acs.org/estengg Review
https://doi.org/10.1021/acsestengg.1c00258
ACS EST Engg. XXXX, XXX, XXX−XXX
D

that are too large to reject the ionic form.
152
RO can reject 60−
98% As(V) and/or 65−80% As(III) in the influent; thus, in
some cases, two or more steps are needed to reach WQOs.
147
If
the source water is anoxic, preoxidation of arsenite to arsenate is
recommended, since at typical pH arsenite is present as a neutral
form, while arsenate is anionic, increasing rejection. However, if
pH adjustment is needed to remove arsenate, it may be more
cost-effective to have two-step RO filtration.
147,148
Sea water (SWRO) and brackish water (BWRO) polyamide
membranes reject boron by 80−93% and 30−80%, respectively.
Consequently, a single-pass RO process is usually unable to
remove boron down to WQOs. Boron removal can be improved
by (a) pre- and post-treatment techniques;
153
(b) double-pass
RO;
153
(c) membrane modification;
149,150
(d) electroactive
membranes.
154
Removal of boron using a second pass or
alternative method increases water cost by approximately 10−
20%.
153
While Br
−
by itself does not pose a concern, the potential
formation of bromate (BrO
3
−
)andotherbrominated
disinfection byproducts (Br-DBPs) during ozone-based dis-
infection processes can be a problem if local surface or
groundwater contains high l evels of Br
−
(e.g., Australia).
While Br
−
removal efficiency of the RO process usually ranges
from 93% to 99.3% when total dissolved salt (TDS)
concentrations are low, it could decline to 74% when TDSs
increased to 400 ppm.
155
In terms of Br-DBPs, their removal
efficiencies in the RO process are relatively low, ranging from 0
(i.e., bromomethane) to 80% (i.e., 1,2-dibromoethane).
156
Moreover, while AOP processes can degrade Br-DBPs, the
risk of forming bromate is quite high. To lower the levels of Br-
DBPs, it is important to remove Br
−
before the AOP process.
Double-pass RO is an option,
157
and new approaches such as
Br
−
selective ion-exchange resin and membrane capacitive
deionization are being explored.
157,158
Low Molecular Mass Volatile, Semivolatile, and
Nonvolatile Organic Compounds. The (secondary) bio-
logical treatment in a WWTP can efficiently remove many
volatile organic compounds (VOCs) or semi-VOCs (e.g., 96.7 ±
1.6% of 2-butanone, 91.7 ± 5.9% of acetone), but the
degradation of halogenated VOCs declines significantly and
may be even nil (e.g., chloroform, cis-1,3-dichloropropene, and
1,2-dichloroethane).
159
Some may be lost to the atmosphere,
and a reaeration step may help lower their concentrations. VOCs
are poorly rejected by RO due to their small molecular mass and
neutral charge.
156
For instance, the rejection ratios of
acetonitrile, acrylonitrile, ch loroethane, 1,1-dich loroethene,
and trichloroethene are 23 ± 10.6%, 18 ± 8.1%, 15 ± 3.6%,
17 ± 9%, and 46 ± 2.9%, respectively.
156
Moreover, membrane
fouling caused by many VOCs significantly decreases water flux.
For instance, fouling caused by hydroquinone, 4-nitrophenol,
and 4-chlorophenol reduced permeate water flux by 2.7%, 4.8%,
and 8.6%, respectively,
160
indicating that high loads of these
pollutants may deteriorate membrane performance.
GAC and other porous materials (e.g., zeolites) can remove
most organic compounds to a large extent, particularly if it is
biologically active.
161−163
VOCs can be removed by inter-
ception, hydrophobic interactions, electrostatic interaction,
multiple hydrogen bo nding, and various types of π ·· ·Cl
interactions.
161,162
The massive number of adsorption sites on
the huge surface areas of these porous materials are responsible
for the large removal capacity and nanoadsorbents are in
development for specific classes of pollutants.
164,165
Biofilms can
form on the GAC or other adsorbents to further increase the
removal capacity of many halogenated VOCs (e.g., methylene
chloride, chlorobenze ne, carbon tetrachloride, tetrachloro-
ethylene, 95% o-chlorophenol, trichloroethene, cis -1,2-dichloro-
ethylene, trans-1,2-DCE, and vinyl chloride).
166,167
Removal
and destruction of volatile and semivolatile organic compounds
can be enhanced by combining it with ozone, since activated
carbon can serve to produce hydroxyl radicals from ozone.
168
This can also remove many of the precursors to disinfection
byproducts. To increase the effectiveness, combinations of
AOPs with GAC, such as O
3
+ GAC + UV/H
2
O
2
, can be used to
enhance the destruction of the organics.
169
The most cost-
effective configuration for many organics was O
3
+ GAC +
O
3
.
169
Photoreactors, in some cases using nano-TiO
2
and UV,
are being considered as alternatives for the degradation of
challenging organics.
170
Many PPCPs can be removed by these
processes, either GAC alone or i n combination with
AOPs.
171−174
PFASs can also be removed to a certain extent
with GAC, although short-chain PFASs exhibited desorption,
and branched PFASs and those with carboxylic acids exhibited
lower adsorption than PFASs that are linear or contain sulfonic
acids.
175
Electrochemical systems can also function as advanced
oxidation/reduction processes for VOC degradation. Over
80% of chloroform, benzene, toluene, and trichloroethene in
solution can be oxidized within 2 h on Ir/Pd doped titanium
electrodes.
176
In addition, halogenated VOCs can be efficiently
electrochemically reduced to halogen-free products on different
types of cathodes at relatively low potentials from −0.3 to −1.4
V (versus standard hydrogen electrode).
177,178
Thus, heteroge-
neous AOP processes, including the electrochemical system, are
increasingly widely adopted for organic pollutant removal.
179,180
In these processes, the reduction/oxidation rate is positively
correlated to the surface areas of the catalysts; thus, the
application of nanoscale materials becomes attractive.
180
The
introduction of nanomaterials not only concentrates the trace
pollutants on electrodes but also delivers electrons onto
adsorbed pollutants, which can significantly increase pollutant
removal performance and energy efficacy as well.
181,182
In the
future, the heterogeneous AOP process for VOC degradation
may play an important role in DPR.
Two m olecules pose partic ular challenges, namely, N-
nitrosodimethylamine (NDMA) and 1,4-dioxane. NDMA
(74.08 Da), both a former industrial chemical and a
chloramination byproduct, is detected in drinking water and
wastewater treatment plants, typically at levels below 100 ng/
L,
183−185
but in industrial areas, concentrations up to 8230 ng/L
have been observed.
186
The USEPA screening level is 0.42 ng/L.
The removal efficiency of NDMA and other nitrosamines in
conventional WWTP biological processes ranges from 0% to
96% from plant to plant and, even the same plant, can experience
a wide range of removal,
183,184
making it particularly challenging
for DPR. The minimization of nitrite yield in the biological
process and/or the addition of sand filtration after secondary
sedimentation can reduce NDMA levels in secondary treatment
effluent,
183,187
but it still remains as a challenge to fully eliminate
it through biological treatment. Due to the existence of NDMA
precursors in secondary effluent, it is very likely that NDMA and
other nitrosamines would be generated again during disinfection
if they are not fully removed before this step.
Treatment options, before feeding the secondary treatment
effluent into an RO system, include filtration (i.e., sand filtration,
GAC adsorption, and nanofiltration) and coagulation/floccu-
lation, which all exhibit limited to moderate removal capacity for
ACS ES&T Engineering pubs.acs.org/estengg Review
https://doi.org/10.1021/acsestengg.1c00258
ACS EST Engg. XXXX, XXX, XXX−XXX
E
NDMA (16−44% with an NDMA concentration ranging
between 14 and 370 ng/L) and NDMA precursors.
188
Removal
efficiency of these processes varied widely depending on raw
water qualities and the speci fic biological treatment process. In
fact, studies report that coagulant dosing and biofiltration can
contribute to the formation of NDMA precursors.
188
Nano-
filtration (NF) can remove up to 90% of NDMA precursors,
189
but there could be significant leaching of NDMA precursors
(∼180−450 ng/L in permeate) after membrane fouling.
189
Due to its small molecular radius (0.248 nm), high
hydrophilicity, and neutral charge at pH 6−8, NDMA is not
effectively removed by RO membranes.
190,191
To enhance
NDMA removal, strategies such as (a) heat treatment of three
types of RO membranes (HYDRA, ESPAB, and ESPA2)
improved NDMA rejection from 74%, 62%, and 53% to 88%,
79%, and 62%, respectively;
192
(b) plugging the RO membrane
with dodecylamine increased rejection from 42% to 81%;
193
(c)
modification of RO with graphene oxide nanosheets enhanced
NDMA rejection from 76.5% to 82.7%.
194
However, while these
strategies increased NDMA rejection, the trade-off was that the
membranes exhibited lower permeability.
194
These strategies
are still in the research stage, and with current RO membranes,
there is no guarant ee that NDMA would be complet ely
removed; for instance, it was reported that the concentration
of NDMA in RO permeate ranged from 8.8 to 31 ng/L.
195
Moreover, the rejection of NDMA precursors declined with
increasing membrane age and after membrane cleaning; the
NDMA rejection ratio decreased 6−9% during the first 4 h
before increasing back to the precleaning rejection ratio.
196,197
In terms of AOPs, H
2
O
2
, ozone, and ClO
2
alone decrease the
concentrations of NDMA and NDMA precursors to some
degree, but the potential formation of NDMA in subsequent
chloramination and its partial rejection of RO highlight the need
for a combined AOP.
198,199
Since the removal efficiencies of
NDMA using UV + H
2
O
2
,UV+O
3
, and UV + monochloramine
are all above 95% and that using UV/free chlorine is 81− 95%,
UV-based technologies are favored.
195
However, excessive UV
radiation and oxidant dosage can increase NDMA formation
potential.
96
1,4-Dioxane (88.11 Da) is a stabilizer added to chlorinated
organic solvents, which is found as a contaminant in some urban
groundwater basins.
72
It is also poorly rejected by NF and RO
due to its high polarity and small size.
200,201
Traditional AOPs
such as O
3
or UV + H
2
O
2
are not sufficiently effective to remove
it from the RO permeate.
202,203
Thus, research is ongoing to
achieve higher removal efficiencies for 1,4-dioxane, such as
reductive electrochemical activation of hydrogen peroxide.
203
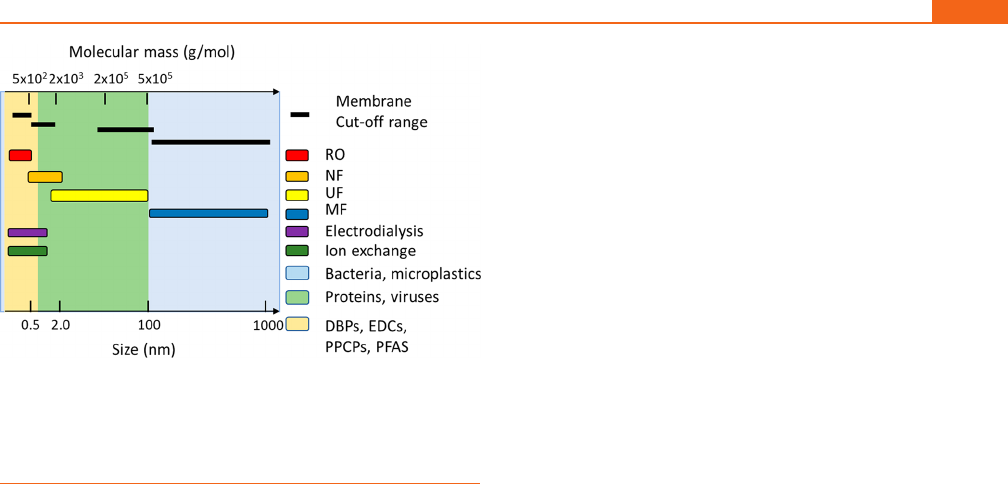
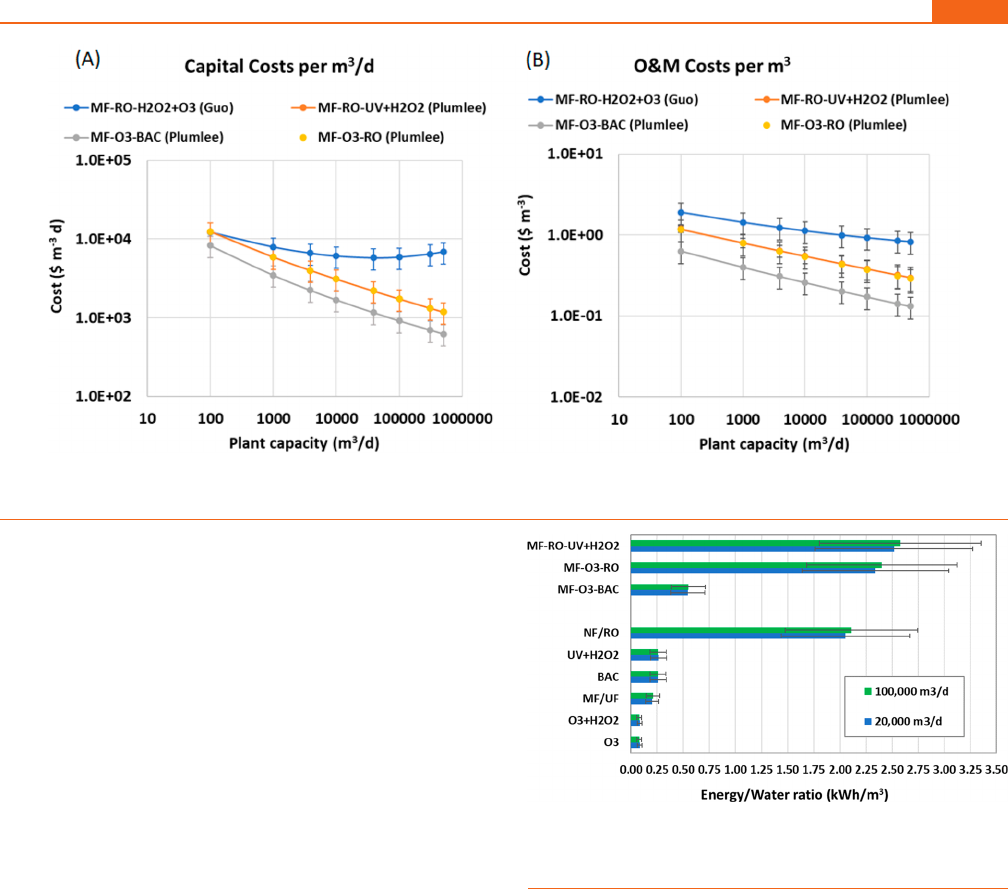
Figure 3. Estimated (A) total capital costs; (B) annual operating expenses; (C) capital costs per 1000 m
3
of capacity; (D) operating annual expenses
per 1000 m
3
. From Guo et al.: RO = reverse osmosis, UF/MF = ultrafiltration/microfiltration, MBR = membrane bioreactor, AS = activated sludge,
COAG = coagulation, H
2
O
2
+O
3
(1) = peroxone, and GAC = granular activated carbon.
9
From Plumlee et al.: NF = nanofiltration, UV + H
2
O
2
=
ultraviolet + hydrogen peroxide, O
3
= ozone, BAC = biologically activated carbon, and H
2
O
2
+O
3
(2) = peroxone.
218
All costs adjusted for inflation to
2021.
ACS ES&T Engineering pubs.acs.org/estengg Review
https://doi.org/10.1021/acsestengg.1c00258
ACS EST Engg. XXXX, XXX, XXX−XXX
F

BAC and O
3
−BAC may also be cost-effective approaches for
1,4-dioxane.
204,205
Pathogens. Pathogens, including viruses, have a particle size
large than 10 nm,
206
and thus, RO should be able to achieve
100% removal based on size exclusion theory.
207−209
Forward
osmosis followed by RO treatment was reported to achieve 6.7-
log removal of spiked MS2 bacteriophage in graywater and
sewage, 5.4-log removal of native Escherichia coli (E. coli)in
graywater, and 7.9-log removal of native E. coli in sewage.
210
However, regrowth of the native bacteria in RO permeate was
still observed, indicating that additional disinfection is
needed.
210
In addition, monitoring virus removal after RO still
presents challenges.
211,212
Thus, UV, O
3
, and/or chlorination
treatment is highly recommended after RO. For instance, dosing
sodium hypochlorite can completely eliminate SARS-CoV-2 in
septic tank wastewater.
213
■
UNIT PROCESSES AND TREATMENT TRAIN COST
ESTIMATES
There are many combinations of advanced tertiary treatment
trains that can be considered for a given location for DPR,
29,214
on the basis of local water sources and conditions, existing
WWTP infrastructure and space, availability of brine disposal to
the ocean vs inland, energy costs, funding mechanisms, etc.
These factors as well as the selected treatment train, scale (i.e.,
flow rate), and local regulatory requirements can substantially
affect the final installed capital and operating costs. Nevertheless,
it is useful to consider the cost of the various treatment units to
plan for DPR to make an informed decision about the various
water supply options. A particular challenge in obtaining cost
information is that most publications are based on small-scale
lab studi es or a relatively limited number of large-scale
operations with very different conditions.
214−216
Guo et al.
conducted a very detailed study of the capital (CAPEX) and
operating (OPEX) expenses of many of the unit operations (e.g.,
RO, UF + MF, membrane bioreactor (MBR), activated sludge
(AS), coagulation (COAG), peroxone (H
2
O
2
+O
3
), and
granular activated carbon (GAC)) and generated scaling
equations.
9,217
Figure 3 presents the inflation-adjusted CAPEX
(Figure 3A) and OPEX (Figure 3B) costs (from 2012 to 2021)
based on equations for plant capacities ranging from 100 to
500 000 m
3
/d. Chlorination is expected to be a very small
fraction of the total cost of the DPR train.
217
To complement the
information, the cost equations developed by Plumlee et al. were
also included in Figure 3, which were also adjusted for inflation
(from 2011 to 2021).
218
These authors consider that the
CAPEX and OPEX for RO are exactly the same as for NF (thus,
they are not presented in Figure 3). In terms of overall capital
costs, the capital investment per unit operation for a 100 000
m
3
/d (∼26.4 million gallons per day) plant ranges from around
$10M to $162M, excluding coagulation and peroxone treat-
ment,
111
as estimated using the information from Guo et al.
9
Most IPR and DPR plants built to date have a capacity of around
100 000 m
3
/d.
219
While in general the cost functions from Guo
et al.
9
and Plumlee et al.
218
are comparable in their estimates for
a given technology, there is a major discrepancy in the estimates
for peroxone (H
2
O
2
+O
3
), CAPEX, and OPEX. Both equations
used to estimate this are presented in Figure 3. The data used for
Figure 3 is provided in Tables S1 and S2.
On the basis of these equations, at 100 000 m
3
/d, CAPEX is
ranked as follows: COAG ≪ O
3
=H
2
O
2
+O
3
(Plumlee et al.
218
)
=UV+H
2
O
2
< GAC < BAC < RO < UF/MF < AS < NF < MBR
<H
2
O
2
+O
3
(Guo et al.
9
); OPEX is ranked as O
3
<H
2
O
2
+O
3
(Plumlee et al.
218
)<UV+H
2
O
2
< BAC < AS < COAG < GAC <
UF/MF = MBR < RO < NF < H
2
O
2
+O
3
(Guo et al.
9
). These
cost estimates have an uncertainty of −30/+50%, and local
conditions may result in significant differences. Thus, there
could be substantial overlap in the range of estimates for the
various technologies, which affect the ranking. Naturally, the
assumptions embedded in the cost estimate equations are
important and may differ for each WWTP. For example, BAC as
estimated here assumes a 10 min
218
empty bed contact time
(EBCT), but other EBCTs may increase or decrease both
CAPEX and OPEX.
218
O
3
is considered pre-RO, while H
2
O
2
+
O
3
is intended to be post-RO. O
3
dosage is considered at 6 mg/L
in the influent, but since some is consumed by the organic
matter, it is estimated at an effective dose of 3 mg/L with a 5 min
hydraulic contact time.
218
The increase of the effective dose of
ozone from 1.5 to 9 mg/L can result in an increase of 8% in
CAPEX for an ∼100 000 m
3
/d plant and 22% increase in
OPEX.
218
Low pressure (e.g., MF/UF) and high pressure (e.g.,
RO, NF) membranes and labor costs can be combined, reducing
overall OPEX.
218
The cost of electricity can be quite significant
as a fraction of OPEX and will differ substantially for each region.
The effect of scale is clearer when CAPEX and OPEX are
normalized by plant capacity (m
3
/d) and annual flow (m
3
),
respectively (Figure 3C,D). CAPEX decreases substantially with
increasing scale for most technologies, except for UV + H
2
O
2
,
which remains near $115 per m
3
/d, and for peroxone as
calculated from the equation in Guo et al.,
9
in which CAPEX first
decreases slowly until the plant capacity increases above
∼10 000 m
3
/d, and then unit costs begin to increase again
with increasing plant capacity. This behavior differs considerably
from the prediction using the equation in Plumlee et al.
218
for
peroxone, which has a continuous decrease in CAPEX with
scale. In terms of OPEX, most technologies exhibit a gradual but
consistent decrease with scale, except GAC and UF/MF, for
which OPEX decreases very rapidly with increasing plant
capacity. However, BAC, a very similar technology to GAC,
appears to reach a flat cost of around $0.045 per m
3
above a plant
capacity of 10 000 m
3
/d.
The CAPEX and OPEX of NF are expected to be similar to
those of RO.
218,220
NF can operate at slightly lower pressure
than RO, which reduces OPEX.
76
The retentate from NF and
RO contains a significant fraction of the CECs that were not fully
removed in the conventional wastewater treatment process and
may require additional treatment before being disposed of, for
example, using AOPs, such as ozone.
220
Since NF and RO have a
recovery ratio of 50−85%,
220
the waste stream to treat and
dispose can be significant. If ozone is used for this treatment,
with or without UV, the transfer efficiency of ozone from the gas
phase where it is generated to the liquid phase needs to be high,
around 70−90%.
221,222
Although some NF can perform very
well compared to RO in the removal of most CECs, they may
not meet the California requirement of TOC < 0.5 mg/L and
also have a poor rejection of nitrate and other monovalent
ions.
76
For example, a study to remove a combination of PFAS
using NF from concentrate estimated OPEX of around $0.25−
0.50/m
3
at a flow rate of 11.5 m
3
/d, depending on the treatment
goal, which is similar to the estimate using the equation in
Plumlee et al.
218
(Figure 3D).
115
Some NF can lower most CEC
concentrations below 100 ng/L and, if bromide is not a concern,
NF can be more economical due to the lower pressure
requirements and fouling potential, which can result in 50%
fewer cleanings and higher overall utilization compared to RO.
76
The cost of fouling is significant and can represent up to 24 ± 3%
ACS ES&T Engineering pubs.acs.org/estengg Review
https://doi.org/10.1021/acsestengg.1c00258
ACS EST Engg. XXXX, XXX, XXX−XXX
G
of OPEX for RO and 11 ± 1% of OPEX for NF.
223
Fouling
results in additional energy, earlier membrane replacement, and
minor additional cleaning costs.
223
Fouling also results in
additional downtime, which can be an important loss of use if
cleaning-in-place events are frequent.
223
Four advanced tertiary treatment trains were considered
(Figure 4), although there are many other possible combina-
tions. The least expensive in terms of CAPEX and OPEX is MF−
O
3
−BAC as proposed by Snyder et al.,
224
which can produce
high effluent quality largely eliminating most CECs, although it
may not achieve TDS treatment goals and thus may be more
suitable for IPR.
224
CAPEX and OPEX are essentially the same
for MF−RO−UV + H
2
O
2
and MF−O
3
−RO,
218
and these two
treatment trains have the potential to achieve very high effluent
quality and meet the strict California requirements. The most
expensive treatment train would be MF−RO−H
2
O
2
+O
3
,
218
which also has the lowest economies of scale. The CAPEX
estimated here corresponds well (within −30/+50%) to those in
operational or pilot-scale plants in the US.
219
In all cases, the
added cost of these advanced tertiary treatment trains will have
an important impact on the overall cost of water for ratepayers.
However, CAPEX for RO and NF continues to decrease with
increasing installed capacity,
225,226
which has not been taken
into consideration in these estimates. On the other hand, we are
approaching the thermodynamic limit of separation
227,228
with
costs for membranes reaching an asymptote.
229
■
ENERGY AND OTHER ENVIRONMENTAL
CONSIDERATIONS
Energy considerations are paramount to potable reuse, given the
significant increase in the energy required per m
3
of treated
water. For the unit operations being considered for potable
reuse, the largest requirement would be from the high-pressure
membranes (Figure 5) followed by the energy requirements for
UV + H
2
O
2
, which are very similar to those of BAC and MF/UF.
As with the cost estimates, these values have an uncertainty of
−30/+50%, and local conditions may result in significant
differences. The range of values also depends on the level of
technology (e.g., older vs newer membranes, ozone generating
system, UV lamps). Thus, treatment trains that consider RO (or
NF) would have 80−90% higher energy requirements than
alternatives such as MF−O
3
−BAC. These estimates based on
modeling equations can be compared to a recent study that
found the electricity intensity in 70 operating, planned, or pilot
fully advanced treatment systems to range from 0.9 to 2.2 kWh/
m
3
with operational systems reporting from 1.1 to 1.4 kWh/
m
3
.
219
To compare the energy required to degrade CECs, it is useful
to consider the concept of electrical energy per order (EE/O, in
kWh/m
3
), which is the electrical energy needed to degrade a
particular chemical by 1 order of magnitude in 1 m
3
of water.
230
A recent review of the EEOs of 13 AOPs for a large number of
molecules concluded that O
3
alone had in general the lowest
median EEO followed by H
2
O
2
+O
3
< electron beam < UV +
chlorine < UV + persulfate < UV + O
3
<UV+H
2
O
2
< photo-
Fenton < plasma.
231
The median EEO values for the first seven
EEOs in the ranking are <1 kWh/m
3
. For photo-Fenton and
plasma, the median EEO values are 3−5 kWh/m
3
. There is a
significant difference in the EEO for the various chemicals,
depending on the molecular structure and physicochemical
properties, in some cases ranging over 5 orders of magnitude
(e.g., from 10
−3
to 10
1
kWh/m
3
for O
3
).
231
EEO is also
dependent on the dose of H
2
O
2
, UV lamp type and arrangement,
and other operating conditions such as the efficiency with which
Figure 4. Integrated advanced tertiary treatment train (A) capital costs; (B) annual operating expenses. The treatment train costs are calculated with
the equations from Guo et al.
9
and Plumlee et al.
218
All costs adjusted for inflation to 2021.
Figure 5. Energy/water ratio for different unit processes as well as three
possible treatment trains for DPR or IPR. Energy data from Plumlee et
al.
218
ACS ES&T Engineering pubs.acs.org/estengg Review
https://doi.org/10.1021/acsestengg.1c00258
ACS EST Engg. XXXX, XXX, XXX−XXX
H

O
3
can be transferred from the gas phase to water.
231−238
EEOs
are also reported only for the AOP process itself and does not
take into account energy requirements for purchased chemicals
or other life-cycle stages.
231
While it is useful to consider EEO, it
is important to note that the removal efficiency generally
decreases as follows: plasma < UV-based AOPs < O
3
-based
AOPs.
239
To select the technology for a given DPR system, one
will need to consider the most recalcitrant contaminants in
wastewater that are not removed by the previous or subsequent
processes in the treatment train.
The advanced tertiary treatment train not only will increase
the energy footprint of the WWTP and the embedded energy
but also, depending on the composition of the electrical grid,
may result in increased emissions of greenhouse gases,
particulate matter of all sizes, mercury, and other air pollutants
as well as its on water demand.
240,241
On the other hand, as the
electrical grid relies more and more on renewable energy, in
general with lower environmental impacts, the increased
energy/water ratio may not result in a much higher environ-
mental footprint. It should also be noted that the transport of
water and wastewater to the corresponding treatment plants
requires a considerable amount of energy, in some cases more
than the energy of conventional wastewater treatment.
242
Domestic water heating also represents a much higher energy
intensity (35−70 kWh/m
3
, depending on inlet and outlet
temperatures as well as fuel source)
243
than those in these
treatment trains, highlighting the need to minimize unnecessary
water heating; proper accounting of the water volumes should be
taken into consideration in these assessments. Since domestic
water heating is controlled by consumers, it is important to
educate them on the potential energy savings that can be
achieved at the consumer level. In addition, there is an
opportunity to use DPR systems as a means to store renewable
energy by using it only when it is cheap and abundant on the grid
(i.e., during peak solar hours during the day in California or at
peak wind hours at night in Texas) and then store the treated
water in the emergency buffer reservoir.
244
Creative thinking can
be used to solve these two challenges (i.e., storing renewable
energy and implementing DPR).
■
CRITICAL ROLE OF SENSING IN DPR
IPR and conventional water treatment systems already monitor
a suite of physical, chemical, and biological contaminants on
both continuous and intermittent schedules.
245
However, since
the DPR process provides little buffer capaci ty (if any),
contaminants that do pass through the treatment train can
rapidly spread throughout the distribution system, exposing
consumers to enhanced risk.
245,246
Therefore, the development
and use of real-time sensing capabilities are critical for the safe
use of DPR, as these capabilities can be used to inform operators
and consumers, in real time, of danger to their drinking water
supply.
247
In addition, this information can be used as input to
automated systems used to control the treatment and
distribution of reclaimed water, which can alert operators,
intensify treatment, shut the process down, and divert
contaminated water from the distribution system. However,
while there is a clear need for real-time sensing and monitoring
capabilities, few real-time sensing platforms are currently used,
particularly for the detection of patho gens, trace organic
pollutants (e.g., PPCPs), and trace metals/metalloids, all of
which are present in wastewater at elevated concentrations and
present risks during DPR.
248
The sensing of DPR treatment train performance can be
separated into two categories: (1) sensing of the performance of
individual steps in the treatment train (e.g., the sensing of RO
rejection); (2) sensing of contaminants of concern in the final
treated water before it is introduced to the distribution system.
In many ways, the evaluation of individual treatment steps is
easier, as there are easily measurable water quality properties
that correlate with the overall performance of each step. When
one considers a common treatment train used in potable reuse
(e.g., MF/UF−RO−AOP), the performance of each one of
these steps can be readily inferred by using off-the-shelf sensing
platforms. For instance, optical turbidity meters and UV-
transmittance measurements that deliver readings in real time
are used to monitor the performance of MF and UF
membranes;
249,250
an increase in permeate turbidity can indicate
damage to the membrane, which would require the membrane
to be pinned (if in a hollow-fiber module) or replaced.
251
For
RO membranes, which are designed to reject all charged species
(including monovalent ions), monitoring permeate conductivity
is a simple way to evaluate RO performance, in real time,
although the sensitivity of this method is rather low;
252,253
since
solution conductivity is highly sensitive to ion concentrations,
damage to the RO membrane will manifest in a measurable
increase in conductivity, informing operators that the RO
process needs attention.
253
Fluorescence excitation−emission
measurements (which can measure the presence of small organic
molecules) are another method to evaluate the integrity of an
RO membrane in near real time.
254
In terms of the performance
of AOPs, there are numerous examples of hydroxyl radical
sensors as well as sensors that evaluate the concentrations of
hydrogen peroxide (used as a hydroxyl radical source in the UV/
H
2
O
2
and O
3
/H
2
O
2
systems). Direct measurement of hydroxyl
radicals (the main reactive component in AOPs) and the
measure of H
2
O
2
disappearance (H
2
O
2
is consumed during
hydroxyl radical g eneration) give a measure of process
performance.
255−257
However, in all the cases mentioned
above, while these measurements can fairly accurately determine
the overall integrity of the different treatment steps, these
measurements say nothing about specific contaminants of
concern. Also, these measurements are sensitive to feedwater
quality. For example, increased turbidity and conductivity of the
feed stream will result in elevated readings in the membrane
permeate streams. Therefore, these measurements must be
conducted in both the feed and permeate streams to properly
evaluate process performance. Another example is the ionic
composition of the feedwater; if there is a change in the
composition (e.g., increased Na
+
or Cl
−
concentrations), this
can lead to a drop in the observed RO rejection and could lead to
an erroneous conclusion that the treatment step is failing.
Various approaches have been explored for the monitoring of
specific contaminants of concern in treated wastewater. These
approaches can be further divided into two categories: (1) the
sensing of specific contaminants; (2) the measurement of bulk
water quality metrics (i.e., surrogate metrics) that are correlated
to the presence of trace contaminants.
29,258
While the first
approach offers a more accurate view of what is in the water, the
large number of potential contaminants makes the utilization of
this approach difficult, as it requires numerous sensors that need
constant updating to keep up with the evolving field of potential
contaminants.
29
Because the second approach relies on
correlations between the presence of easily measurable species
and trace contaminants, this approach is thought to be more
cost-effective (and feasible), albeit while sacrificing specificity.
29
ACS ES&T Engineering pubs.acs.org/estengg Review
https://doi.org/10.1021/acsestengg.1c00258
ACS EST Engg. XXXX, XXX, XXX−XXX
I

However, these surrogate measurements are somewhat “water
specific” and require “tuning” to a local water quality profile.
259
A large amount of effort has gone into the development of
sensors for various pathogens, including viruses, bacteria, and
protozoa; a (non)comprehensive list of such sensors can be
found in a recent review.
260
These sensors operate using
different methods but can be grouped into (i) sensors that
operate by monitoring changes to surfaces (e.g., electrical
properties) when a target pathogen attaches to specific binding
sites, which allows for detection in real time; (ii) sensors that
detect specific pathogens by amplifying DNA; (iii) optical
sensors (e.g., cytometry, fluorescence); (iv) colorimetric
sensors.
260
While many of these sensors are highly accurate
and sensitive, many require some time for the detection process
to take place (e.g., to amplify DNA), making real-time detection
impossible. For sensors that offer results in near real time, these
sensors can become fouled by other aquatic species, such as
dissolved organic matter that can form nonspecific (e.g.,
hydrophobic) interactions with the surface, which can block
binding sites and reduce sensitivity.
Sensors for the detection of specific PPCPs (e.g.,
pharmaceuticals, endocrine disruptors, etc.) have also been
explored. Approaches toward their detection include the
fabrication of electrodes with specific binding sites to these
compounds
261,262
as well as spectroscopic analysis (e.g., using
fluorescence spectroscopy, Raman spectroscopy, and infrared
spectroscopy) of water.
263,264
While these sensors often exhibit
high sensitivity and specificity in pure samples, electrochemical
sensors are prone to fouling, and spectroscopic signals can be
complicated to deconvolute in a mixed sample environment.
That being said, the highly treated water resulting from the
multiple treatment step used for DPR has very low
concentrations of other contaminants, making these detection
methods highly attractive. In terms of metal/metalloid sensing,
many reports have described online sensors for the detection of
arsenic and other heavy metals.
265−268
Many arsenic electro-
chemical sensors have been demonstrated, using such methods
as anodic stripping voltammetry and molecular imprinting of
electrodes.
267
To the best of our knowledge, no online sensors
have been developed for boron. There is likely an interesting
opportunity to combine machine learning methods with
monitoring tools for the rapid and flexible detection of a range
of contaminants.
269−272
Specifically, the ability to train a
computer to deconvolute and decipher the complex spectro-
scopic signals resulting from the analysis of aqueous streams may
prove transformative, as the algorithm can be trained to identify
target contaminants as they emerge. For instance, software using
a convolutional neural network was able to recognize nitrates,
some pharmaceuticals, microplastics, and their additives after
being trained on a large set of spectra collected from a deep-UV
Raman/fluorescence spectroscope.
271
A multivariate model
utilizing machine learning algorithms based on a back-
propagation neural network was successfully developed and
trained to accurately read various spectra collected from soil by
laser-induced breakdown spectroscopy for the detection of trace
element analysis.
273
UV−vis spectroscopic data was used to
train a fitness-support vector machine classifier, which was then
able to serve as an early warning system for water contamination
events.
274
While they require significant amounts of high-quality
data for training, machine learning approaches can offer high
reliability for detecting and monitoring contaminants in
different water matrixes. For instance, after being trained with
a data set of 12 560 UV spectra, a system using machine learning
algorithms successfully detected target contaminants in 107
times out of a total of 109 measurements and did not generate
any false positive signals.
274
The installation of sensor systems will likely increase the
capital costs of water treatment systems. However, a system with
a series of in-line sensors has the potential of not only
minimizing operational failures but also informing data-driven
system control algorithms (using artificial intelligence ap-
proaches) that optimize water treatment processes and reduce
operational costs.
275
For example, it has been demonstrated that
a data-driven approach could reduce pump energy consumption
in a wastewater treatment plant by 18.5%;
276
a model based on
artificial neural networks cut coagulant dosage by 10%,
277
and an
in-line control system built on a genetic algorithm utilizing a
fuzzy wavelet neural network algorithm provided robust and
effective dissolved oxygen control and reduced the demand for
aeration.
278
In addition, it is anticipated that the benefits of in-
line sensor systems coupled to artificial-intelligence optimiza-
tion algorithms will increase the adoption of such sensors, which
will potentially reduce the costs of these sensors, bringing down
the associated capital costs.
■
RECOMMENDATIONS
A number of treatment trains (e.g., MF−RO−AOP, MF−O
3
−
BAC) have been successfully tested (e.g., in pilot tests and IPR
systems) and can produce the high-quality effluent needed for
DPR. Costs and energy requirements continue to decrease for
RO and NF, although thermodynamic limits are being reached,
and a reduction in cost and energy is a technological
challenge.
145
The most significant challenges are in online,
real-time sensing of a number of small molecules that may be
present in the influent; breakthroughs in this area will be needed
for full deployment of DPR to ensure high reliability and
consumer safety. Of particular concern would be high episodic
loads from accidental or unreported discharges to the sewer
system that could move quickly through the treatment system
and pass to the distribution system at concentrations that could
pose a concern as well as the rapidly evolving field of trace
contaminants that find their way through the treatment system
and can be harmful to consumers when chronically present in
the water.
Establish Online and Real-Time Water Quality Mon-
itoring Systems. There have been important advances in water
quality monitoring, but there is a critical need to quantitatively
monitor a number of molecules and conditions, which can serve
as indicators of unit process or treatment train performance.
Monitoring has to have a high d egree of reliability and
redundancy to avoid failures as well as provide warning in real
time to operators and consumers. Since there is little buffering
capacity, rapid measurements are critical to safety. There may be
an attractive opportunity to utilize machine learning approaches
to analyze spectroscopic data (which can be rapidly collected
and analyzed) and inform operators of potential problems.
Redundancy in Treatment Processes to Ensure a High
Level of Removal. If DPR is to get main-stream acceptance
and become a regular water source, redundant treatment
equipment will be needed to handle maintenance as well as load
surges and other emergencies. This will increase the overall
CAPEX. In any case, this will likely be the most expensive water
source for most municipalities, even if CAPEX and OPEX costs,
particularly those of RO/NF membranes, continue to decrease
as more capacity is installed.
ACS ES&T Engineering pubs.acs.org/estengg Review
https://doi.org/10.1021/acsestengg.1c00258
ACS EST Engg. XXXX, XXX, XXX−XXX
J

Emergency Buffer and Plans for Dealing with Off-Spec
Water. If the monitoring system detects a deviation from
specifications or there is a detected equipment failure or under-
performance, there will be a need for an emergency buffer
reservoir to store the off-spec water and a clear process for
dealing with it for either reprocessing or discharge into an
environmental receiving water body without causing undue
environmental damage.
Consideration of Renewable Energy. Most DPR treat-
ment trains will have a substantial impact on the embedded
energy in water. This may become a barrier for adoption, unless
there is a plan to employ renewable energy that will result in
lower emissions of carbon and many pollutants associated with
conventional fuels. Building renewable energy at the same pace
as DPR is implemented must be part of the plan as well as
developing creative ideas for storing renewable energy from the
grid by filling the emergency reservoir with treated water from
DPR. There is also a need to educate the consumer about
opportunities for energy reduction in domestic water use, which
can be as important as water treatment in the overall balance.
■
ASSOCIATED CONTENT
*
sı
Supporting Information
The Supporting Information is available free of charge at
https://pubs.acs.org/doi/10.1021/acsestengg.1c00258.
Two tables with capital and operating expenses for
different unit processes and treatment trains (PDF)
One spreadsheet with equations to calculate capital and
operating expenses (XLSX)
■
AUTHOR INFORMATION
Corresponding Author
Arturo A. Keller − Bren School of Environmental Science and
Management, University of California Santa Barbara, Santa
Barbara, California 93106, United States;
orcid.org/
0000-0002-7638-662X; Email: [email protected]
Authors
Yiming Su − Department of Civil and Environmental
Engineering, University of California Los Angeles, Los Angeles,
California 93106, United States;
orcid.org/0000-0001-
6035-7384
David Jassby − Department of Civil and Environmental
Engineering, University of California Los Angeles, Los Angeles,
California 93106, United States;
orcid.org/0000-0002-
2133-2536
Complete contact information is available at:
https://pubs.acs.org/10.1021/acsestengg.1c00258
Notes
The authors declare no competing financial interest.
■
REFERENCES
(1) Haddeland, I.; Heinke, J.; Biemans, H.; Eisner, S.; Flörke, M.;
Hanasaki, N.; Konzmann, M.; Ludwig, F.; Masaki, Y.; Schewe, J.;
Stacke, T.; Tessler, Z. D.; Wada, Y.; Wisser, D. Global Water Resources
Affected by Human Interventions and Climate Change. Proc. Natl.
Acad. Sci. U. S. A. 2014, 111 (9), 3251−3256.
(2) Papa, M.; Foladori, P.; Guglielmi, L.; Bertanza, G. How Far Are
We from Closing the Loop of Sewage Resource Recovery? A Real
Picture of Municipal Wastewater Treatment Plants in Italy. J. Environ.
Manage. 2017, 198,9−15.
(3) Sedlak, D. Water 4.0, The Past, Present, and Future of the World’s
Most Vital Resource; Yale University Press, 2015.
(4) Seah, H.; Tan, T. P.; Chong, M. L.; Leong, J. NEWater-Multi
Safety Barrier Approach for Indirect Potable Use. Water Sci. Technol.:
Water Supply 2008, 8 (5), 573−582.
(5) Lee, H.; Tan, T. P. Singapore’s Experience with Reclaimed Water:
NEWater. Int. J. Water Resour. Dev. 2016, 32 (4), 611−621.
(6) Marron, E. L.; Mitch, W. A.; von Gunten, U.; Sedlak, D. L. A Tale
of Two Treatments: The Multiple Barrier Approach to Removing
Chemical Contaminants during Potable Water Reuse. Acc. Chem. Res.
2019, 52 (3), 615−622.
(7) Onyango, L.; Leslie, G.; Wood, J. G. Global Potable Reuse Case
Study 2: Upper Occoquan Service Authority; Brisbane, Australia, 2014.
(8) Apostolidis, N.; Hertle, C.; Young, R. Water Recycling in Australia.
Water (Basel, Switz.) 2011, 3 (3), 869−881.
(9) Guo, T.; Englehardt, J.; Wu, T. Review of Cost versus Scale: Water
and Wastewater Treatment and Reuse Processes. Water Sci. Technol.
2014, 69 (2), 223−234.
(10) USEPA. Guidelines for Water Reuse; EPA/600/R-12/618;
USEPA, 2012.
(11) Schimmoller, L. J.; Kealy, M. J.; Foster, S. K. Triple Bottom Line
Costs for Multiple Potable Reuse Treatment Schemes. Environ. Sci.
Water Res. Technol. 2015, 1 (5), 644−658.
(12) Wester, J.; Broad, K. Direct Potable Water Recycling in Texas:
Case Studies and Policy Implications. J. Environ. Policy Plan. 2021, 23
(1), 66−
83.
(13) Sahin, O.; Bertone, E.; Beal, C. D. A Systems Approach for
Assessing Water Conservation Potential through Demand-Based Water
Tariffs. J. Cleaner Prod. 2017, 148, 773−784.
(14) Gonzales, P.; Ajami, N. Social and Structural Patterns of
Drought-Related Water Conservation and Rebound. Water Resour. Res.
2017, 53 (12), 10619−10634.
(15) Corral-Verdugo, V.; Carrus, G.; Bonnes, M.; Moser, G.; Sinha, J.
B. P. Environmental Beliefs and Endorsement of Sustainable Develop-
ment Principles in Water Conservation: Toward a New Human
Interdependence Paradigm Scale. Environ. Behav. 2008, 40 (5), 703−
725.
(16) Clark, W. A.; Finley, J. C. Determinants of Water Conservation
Intention in Blagoevgrad, Bulgaria. Soc. Nat. Resour. 2007, 20 (7), 613−
627.
(17) Aprile, M. C.; Fiorillo, D. Water Conservation Behavior and
Environmental Concerns: Evidence from a Representative Sample of
Italian Individuals. J. Cleaner Prod. 2017, 159, 119−129.
(18) Tang, X.; Jin, Y.; Feng, C.; McLellan, B. C. Optimizing the
Energy and Water Conservation Synergy in China: 2007−2012. J.
Cleaner Prod. 2018, 175,8−17.
(19) Goette, L.; Leong, C.; Qian, N. Motivating Household Water
Conservation: A Field Experiment in Singapore. PLoS One 2019, 14
(3), No. e0211891.
(20) Inman, D.; Jeffrey, P. A Review of Residential Water
Conservation Tool Performance and Influences on Implementation
Effectiveness. Urban Water J. 2006, 3, 127−143 September.
(21) Cooley, H.; Phurisamban, R. The Cost of Alternative Water Supply
and Efficiency Options in California; Pacific Institute: Oakland, CA,
2016.
(22) Diringer, S.; Shimabuku, M. Stacked Incentives: Co-Funding Water
Customer Incentive Programs; Pacific Institute: Oakland, CA, 2021.
(23) Sen, S.; Lau, A.; Olney, B.; Ding, L.; Semper, J. P.; Russell, P.
Evaluation of Surface Water Augmentation at Lake Jennings. In Water
Environment Federation Technical Exhibition and Conference 2017,
WEFTEC 2017; Water Environment Federation, 2017; Vol. 3,pp
2119−
2132; DOI: 10.2175/193864717822153715.
(24) Gerrity, D.; Pecson, B.; Trussell, R. S.; Trussell, R. R. Potable
Reuse Treatment Trains throughout the World. Aqua 2013, 62 (6),
321−338.
(25) Pecson, B. M.; Trussell, R. S.; Triolo, S. C.; Trussell, R. R.
Examining Reservoirs in Potable Reuse, Part 1: Groundwater Recharge
and Surface Water Augmentation. J. - Am. Water Works Assoc. 2018, 110
(8), 34−40.
ACS ES&T Engineering pubs.acs.org/estengg Review
https://doi.org/10.1021/acsestengg.1c00258
ACS EST Engg. XXXX, XXX, XXX−XXX
K

(26) Haak, L.; Sundaram, V.; Pagilla, K. Sustainability Assessment for
Indirect Potable Reuse: A Case Study from Reno, Nevada. Water
Environ. Res. 2018, 90 (8), 748−760.
(27) California State Water Resources Control Board. California
Drinking Water-Related Laws; https://www.waterboards.ca.gov/
drinking_water/certlic/drinkingwater/Lawbook.html (accessed June
15, 2021).
(28) Lahnsteiner, J.; van Rensburg, P.; Esterhuizen, J. Direct Potable
Reuse - A Feasible Water Management Option. J. Water Reuse Desalin.
2018, 8 (1), 14−28.
(29) Leverenz, H. L.; Tchobanoglous, G.; Asano, T. Direct Potable
Reuse: A Future Imperative. J. Water Reuse Desalin. 2011, 1 (1), 2−10.
(30)Drewes,J.E.;Hu
̈
bner, U.; Zhiteneva, V.; Karakurt, S.
Characterization of Unplanned Water Reuse in the EU; Garching,
Germany, 2017.
(31) Voulvoulis, N. Water Reuse from a Circular Econ omy
Perspective and Potential Risks from an Unregulated Approach. Curr.
Opin. Environ. Sci. Health 2018, 2,32−45.
(32) Binz, C.; Razavian, N. B.; Kiparsky, M. Of Dreamliners and
Drinking Water: Developing Risk Regulation and a Safety Culture for
Direct Potable Reuse. Water Resour. Manag. 2018, 32 (2), 511−525.
(33) Ahmed, W.; Angel, N.; Edson, J.; Bibby, K.; Bivins, A.; O’Brien, J.
W.; Choi, P. M.; Kitajima, M.; Simpson, S. L.; Li, J.; Tscharke, B.;
Verhagen, R.; Smith, W. J. M.; Zaugg, J.; Dierens, L.; Hugenholtz, P.;
Thomas, K. V.; Mueller, J. F. First Confirmed Detection of SARS-CoV-
2 in Untreated Wastewater in Australia: A Proof of Concept for the
Wastewater Surveillance of COVID-19 in the Community. Sci. Total
Environ. 2020, 728, 138764.
(34) Baldovin, T.; Amoruso, I.; Fonzo, M.; Buja, A.; Baldo, V.;
Cocchio, S.; Bertoncel lo, C . SARS-CoV-2 RNA De tection and
Persistence in Wastewater Samples: An Experimental Network for
COVID-19 Environmental Surveillance in Padua, Veneto Region (NE
Italy). Sci. Total Environ. 2021, 760, 143329.
(35) Kumar, M.; Patel, A. K.; Shah, A. V.; Raval, J.; Rajpara, N.; Joshi,
M.; Joshi, C. G. First Proof of the Capability of Wastewater Surveillance
for COVID-19 in India through Detection of Genetic Material of
SARS-CoV-2. Sci. Total Environ. 2020, 746, 141326.
(36) Hata, A.; Hara-Yamamura, H.; Meuchi, Y.; Imai, S.; Honda, R.
Detection of SARS-CoV-2 in Wastewater in Japan during a COVID-19
Outbreak. Sci. Total Environ. 2021, 758, 143578.
(37)Venugopal,A.;Ganesan,H.;SudalaimuthuRaja,S.S.;
Govindasamy, V.; Arunachalam, M.; Narayanasamy, A.; Sivaprakash,
P.; Rahman, P. K. S. M.; Gopalakrishnan, A. V.; Siama, Z.; Vellingiri, B.
Novel Wastewater Surveillance Strategy for Early Detection of
Coronavirus Disease 2019 Hotspots. Curr. Opin. Environ. Sci. Health
2020, 17,8−13.
(38) McCall, C.; Wu, H.; O’Brien, E.; Xagoraraki, I. Assessment of
Enteric Viruses during a Hepatitis Outbreak in Detroit MI Using
Wastewater Surveillance and Metagenomic Analysis. J. Appl. Microbiol.
2021, 131 , 1539.
(39) Hou, C.; Hua, Z.; Xu, P.; Xu, H.; Wang, Y.; Liao, J.; Di, B.
Estimating the Prevalence of Hepatitis B by Wastewater-Based
Epidemiology in 19 Cities in China. Sci. Total Environ. 2020 , 740 ,
139696.
(40) Bibby, K.; Aquino De Carvalho, N.; Wigginton, K. Research
Needs for Wastewater Handling in Virus Outbreak Response. Environ.
Sci. Technol. 2017, 2534−2535.
(41) Bibby, K.; Fischer, R. J.; Casson, L. W.; Stachler, E.; Haas, C. N.;
Munster, V. J. Persistence of Ebola Virus in Sterilized Wastewater.
Environ. Sci. Technol. Lett. 2015, 2 (9), 245−249.
(42) Abu Ali, H.; Yaniv, K.; Bar-Zeev, E.; Chaudhury, S.; Shagan, M.;
Lakkakula, S.; Ronen, Z.; Kushmaro, A.; Nir, O. Tracking SARS-CoV-2
RNA through the Wastewater Treatment Process. ACS ES&T Water
2021, 1 (5), 1161−1167.
(43) Pecson, B. M.; Trussell, R. S.; Pisarenko, A. N.; Trussell, R. R.
Achieving Reliability in Potable Reuse: The Four Rs. J. - Am. Water
Works Assoc. 2015, 107 (3), 48−58.
(44) Stanford, B. D.; Becker, W. C.; Debroux, J. F.; Ishii, S. K. L.;
Khan, S. J.; Khunjar, W. O. Planning for Direct Potable Reuse:
Operational Aspects of an Integrated Drinking Water System. J. - Am.
Water Works Assoc. 2016, 108 (4), 48−55.
(45) Heo, J.; Kim, S.; Her, N.; Park, C. M.; Yu, M.; Yoon, Y. Removal
of Contaminants of Emerging Concern by FO, RO, and UF Membranes
in Water and Wastewater. In Contaminants of Emerging Concern in
Water and Wastewater; Elsevier Inc., 2020; DOI: 10.1016/B978-0-12-
813561-7.00005-5.
(46) Park, M.; Snyder, S. A. Attenuation of Contaminants of Emerging
Concerns by Nano Fi Ltration Membrane: Rejection Mechanism and
Application in Water Reuse. In Contaminants of Emerging Concern in
Water and Wastewater; Elsevier Inc., 2020; DOI: 10.1016/B978-0-12-
813561-7.00006-7.
(47) Teodosiu, C.; Gilca, A. F.; Barjoveanu, G.; Fiore, S. Emerging
Pollutants Removal through Advanced Drinking Water Treatment: A
Review on Processes and Environmental Performances Assessment.
J.
Cleaner Prod. 2018, 197, 1210−1221.
(48) Khan, J. A.; Sayed, M.; Khan, S.; Shah, N. S. Advanced Oxidation
Processes for the Treatment of Contaminants of Emerging Concern. In
Contaminants of Emerging Concern in Water and Wastewater; Elsevier
Inc., 2020; DOI: 10.1016/B978-0-12-813561-7.00009-2.
(49) Cartagena, P.; El Kaddouri, M.; Cases, V.; Trapote, A.; Prats, D.
Reduction of Emerging Micropollutants, Organic Matter, Nutrients
and Salinity from Real Wastewater by Combined MBR-NF/RO
Treatment. Sep. Purif. Technol. 2013, 110, 132−143.
(50)Snyder,S.A.;Westerhoff,P.;Yoon,Y.;Sedlak,D.L.
Pharmaceuticals, Personal Care Products, and Endocrine Disruptors
in Water: Implications for the Water Industry. Environ. Eng. Sci. 2003,
20, 449−469.
(51) Vandenberg, L. N.; Colborn, T.; Hayes, T. B.; Heindel, J. J.;
Jacobs, D. R.; Lee, D. H.; Shioda, T.; Soto, A. M.; vom Saal, F. S.;
Welshons, W. V.; Zoeller, R. T.; Myers, J. P. Hormones and Endocrine-
Disrupting Chemicals: Low-Dose Effects and Nonmonotonic Dose
Responses. Endocr. Rev. 2012, 33, 378−455.
(52) Leung, H. W.; Jin, L.; Wei, S.; Tsui, M. M. P.; Zhou, B.; Jiao, L.;
Cheung,P.C.;Chun,Y.K.;Murphy,M.B.;Lam,P.K.S.
Pharmaceuticals in Tap Water: Human Health Risk Assessment and
Proposed Monitoring Framework in China. Environ. Health Perspect.
2013, 121 (7), 839−846.
(53) Xu, Z.; Liu, J.; Wu, X.; Huang, B.; Pan, X. Nonmonotonic
Responses to Low Doses of Xenoestrogens: A Review. Environ. Res.
2017, 155, 199−207.
(54) Lin, T.; Yu, S.; Chen, W. Occurrence, Removal and Risk
Assessment of Pharmaceutical and Personal Care Products (PPCPs) in
an Advanced Drinking Water Treatment Plant (ADWTP) around
Taihu Lake in China. Chemosphere 2016, 152,1−9.
(55) Jenkins, S.; Wang, J.; Eltoum, I.; Desmond, R.; Lamartiniere, C.
A. Chronic Oral Exposure to Bisphenol a Results in a Nonmonotonic
Dose Response in Mammary Carcinogenesis and Metastasis in Mmtv-
Erbb2Mice. Environ. Health Perspect. 2011, 119 (11), 1604−1609.
(56) Riva, F.; Castiglioni, S.; Fattore, E.; Manenti, A.; Davoli, E.;
Zuccato, E. Monitoring Emerging Contaminants in the Drinking Water
of Milan and Assessment of the Human Risk. Int. J. Hyg. Environ. Health
2018, 221 (3), 451−457.
(57) Storey, M. V.; van der Gaag, B.; Burns, B. P. Advances in On-Line
Drinking Water Quality Monitoring and Early Warning Systems. Water
Res. 2011, 45 (2), 741−747.
(58) Soller, J. A.; Eftim, S. E.; Warren, I.; Nappier, S. P. Evaluation of
Microbiological Risks Associated with Direct Potable Reuse. Microb.
Risk Anal. 2017, 5,3−14.
(59) DeCory, T. R.; Durst, R. A.; Zimmerman, S. J.; Garringer, L. A.;
Paluca, G.; DeCory, H. H.; Montagna, R. A. Development of an
Immunomagnetic B ead-I mmunoliposome Fluorescence Assay for
Rapid Detection of Escherichia Coli O157:H7 in Aqueous Samples
and Comparison of the Assay with a Standard Microbiological Method.
Appl. Environ. Microbiol. 2005, 71 (4), 1856−1864.
(60) Connelly, J. T.; Baeumner, A. J. Biosensors for the Detection of
Waterborne Pathogens. Anal. Bioanal. Chem. 2012, 402, 117−127.
(61) Vandegrift, J.; Hooper, J.; da Silva, A.; Bell, K.; Snyder, S.; Rock,
C. M. Overview of Monitoring Techniques for Evaluating Water
ACS ES&T Engineering pubs.acs.org/estengg Review
https://doi.org/10.1021/acsestengg.1c00258
ACS EST Engg. XXXX, XXX, XXX−XXX
L

Quality at Potable Reuse Treatment Facilities. J. - Am. Water Works
Assoc. 2019 , 111 (7), 12−23.
(62) Anumol, T.; Snyder, S. A. Rapid Analysis of Trace Organic
Compounds in Water by Automated Online Solid-Phase Extraction
Coupled to Liquid Chromatography-Tandem Mass Spectrometry.
Talanta 2015, 132,77−86.
(63) Fujioka, T. Removal of N-Nitrosodimethylamine for Potable
Reuse: Reverse Osmosis Treatment and Monitoring Technologies. In
Water and Wastewater Treatment Technologies; Springer Nature, 2019;
pp 167−185; DOI: 10.1007/978-981-13-3259-3_9.
(64) Maruya, K. A.; Wong, C. S. Evaluating Analytical Methods for
Detecting Unknown Chemicals in Recycled Water; https://www.
waterrf.org/research/projects/evaluating-analytical-methods-
detecting-unknown-chemica ls-recycled- water (accessed June 18,
2021).
(65) Ahmad, A.; van der Wens, P.; Baken, K.; de Waal, L.;
Bhattacharya, P.; Stuyfzand, P. Arsenic Reduction to <1 μg/L in
Dutch Drinking Water. Environ. Int. 2020, 134, 105253.
(66) Igra, A. M.; Harari, F.; Lu, Y.; Casimiro, E.; Vahter, M. Boron
Exposure through Drinking Water during Pregnancy and Birth Size.
Environ. Int. 2016, 95,54−60.
(67) DeAngelo, A. B.; George, M. H.; Kilburn, S. R.; Moore, T. M.;
Wolf, D. C. Carcinogenicity of Potassium Bromate Administered in the
Drinking Water to Male B6C3F1Mice and F344/N Rats. Toxicol.
Pathol. 1998, 26 (5), 587−594.
(68) Crofton, K. M. Bromate: Concern for Developmental Neuro-
toxicity? Toxicology 2006, 221 (2−3), 212−216.
(69) Ali, S. N.; Ansari, F. A.; Arif, H.; Mahmood, R. Sodium Chlorate
Induces DNA Damage an d DNA-Pro tein Cross-Linking in Rat
Intestine: A Dose Dependent Study. Chemosphere 2017, 177, 311−316.
(70) Aranda-Rodriguez, R.; Lemieux, F.; Jin, Z.; Hnatiw, J.; Tugulea,
A. M. (Yet More) Challenges for Water Treatment Plants: Potential
Contribution of Hypochlorite Solutions to Bromate, Chlorate, Chlorite
and Perchlorate in Drinking Water. Aqua 2017, 66 (8), 621−631.
(71) Hebert, A.; Forestier, D.; Lenes, D.; Benanou, D.; Jacob, S.; Arfi,
C.; Lambolez, L.; Levi, Y. Innovative Method for Prioritizing Emerging
Disinfection By-Products (DBPs) in Drinking Water on the Basis of
Their Potential Impact on Public Health.
Water Res. 2010, 44 (10),
3147−3165.
(72) McElroy, A. C.; Hyman, M. R.; Knappe, D. R. U. 1,4-Dioxane in
Drinking Water: Emerging for 40 Years and Still Unregulated. Curr.
Opin. Environ. Sci. Health 2019, 7, 117−125.
(73) Young, W. F.; Horth, H.; Crane, R.; Ogden, T.; Arnott, M. Taste
and Odour Threshold Concentrations of Potential Potable Water
Contaminants. Water Res. 1996, 30 (2), 331−340.
(74) Suffet, I. H.; Khiari, D.; Bruchet, A. The Drinking Water Taste
and Odor Wheel for the Millennium: Beyond Geosmin and 2-
Methylisoborneol. Water Sci. Technol. 1999, 40,1−13.
(75) Linge, K. L.; Blair, P.; Busetti, F.; Rodriguez, C.; Heitz, A.
Chemicals in Reverse Osmosis-Treated Wastewater: Occurrence,
Health Risk, and Contribution to Residual Dissolved Organic Carbon.
Aqua 2012 , 61 (8), 494−505.
(76) Bellona, C.; Heil, D.; Yu, C.; Fu, P.; Drewes, J. E. The Pros and
Cons of Using Nanofiltration in Lieu of Reverse Osmosis for Indirect
Potable Reuse Applications. Sep. Purif. Technol. 2012, 85,69−76.
(77) Tabacova, S.; Kimmel, C. A.; Wall, K.; Hansen, D. Atenolol
Developmental Toxicity: Animal-to-Human Comparisons. Birth
Defects Res., Part A 2003, 67 (3), 181−192.
(78) Zhao, Y.; Anichina, J.; Lu, X.; Bull, R. J.; Krasner, S. W.; Hrudey,
S. E.; Li, X. F. Occurrence and Formation of Chloro- and Bromo-
Benzoquinones during Drinking Water Disinfection. Water Res. 2012,
46 (14), 4351−4360.
(79) Santhi, V. A.; Sakai, N.; Ahmad, E. D.; Mustafa, A. M. Occurrence
of Bisphenol A in Surface Water, Drinking Water and Plasma from
Malaysia with Exposure Assessment from Consumption of Drinking
Water. Sci. Total Environ. 2012, 427−428, 332−338.
(80) Chu, I.; Villeneuve, D. C.; Secours, V. E.; Becking, G. C.; Valli, V.
E. Toxicity of Trihalomethanes: I the Acute and Subacute Toxicity of
Chloroform, Bromodichloromethane, Chlorodibromomethane and
Bromoform in Rats. J. Environ. Sci. Health, Part B 1982, 17 (3), 205−
224.
(81) Khallef, M.; Liman, R.; Konuk, M.; Ciierci, I
̇
. H.; Benouareth, D.;
Tabet, M.; Abda, A. Genotoxicity of Drinking Water Disinfection By-
Products (Bromoform and Chloroform) by Using Both Allium
Anaphase-Telophase and Comet Tests. Cytotechnology 2015, 67 (2),
207−213.
(82) Mcdowell, D. C.; Huber, M. M.; Wagner, M.; Von Gunten, U.;
Ternes, T. A. Ozonation of Carbamazepine in Drinking Water:
Identification and Kinetic Study of Major Oxidation Products. Environ.
Sci. Technol. 2005, 39 (20), 8014−8022.
(83) Kumar, A.; Xagoraraki, I. Human Health Risk Assessment of
Pharmaceuticals in Water: An Uncertainty Analysis for Meprobamate,
Carbamazepine, and Phenytoin. Regul. Toxicol. Pharmacol. 2010, 57
(2−3), 146−156.
(84) Houeto, P.; Carton, A.; Guerbet, M.; Mauclaire, A. C.; Gatignol,
C.; Lechat, P.; Masset, D. Assessment of the Health Risks Related to the
Presence of Drug Residues in Water for Human Consumption:
Application to Carbamazepine. Regul. Toxicol. Pharmacol. 2012, 62 (1),
41−48.
(85) Moro, S.; Chipman, J. K.; Antczak, P.; Turan, N.; Dekant, W.;
Falciani, F.; Mally, A. Identification and Pathway Mapping of Furan
Target Proteins Reveal Mitochondrial Energy Production and Redox
Regulation as Critical Targets of Furan Toxicity. Toxicol. Sci. 2012, 126
(2), 336−352.
(86) Prasse, C.; Ford, B.; Nomura, D. K.; Sedlak, D. L. Unexpected
Transformation of Dissolved Phenols to Toxic Dicarbonyls by
Hydroxyl Radicals and UV Light. Proc. Natl. Acad. Sci. U. S. A. 2018,
115 (10), 2311−2316.
(87) Conley, J. M.; Evans, N.; Mash, H.; Rosenblum, L.; Schenck, K.;
Glassmeyer, S.; Furlong, E. T.; Kolpin, D. W.; Wilson, V. S. Comparison
of in Vitro Estrogenic Activity and Estrogen Concentrations in Source
and Treated Waters from 25 U.S. Drinking Water Treatment Plants. Sci.
Total Environ. 2017, 579, 1610−1617.
(88) Sadaria, A. M.; Labban, C. W.; Steele, J. C.; Maurer, M. M.;
Halden, R. U. Retrospective Nationwide Occurrence of Fipronil and Its
Degradates in U.S. Wastewater and Sewage Sludge from 2001 - 2016.
Water Res. 2019, 155, 465−473.
(89) Kartheek, R. M.; David, M. Assessment of Fipronil Toxicity on
Wistar Rats: A Hepatotoxic Perspective. Toxicol. Reports 2018, 5, 448−
456.
(90) Wert, E. C.; Rosario-Ortiz, F. L.; Drury, D. D.; Snyder, S. A.
Formation of Oxidation Byproducts from Ozonation of Wastewater.
Water Res. 2007, 41 (7), 1481−1490.
(91) Papageorgiou, A.; Voutsa, D.; Papadakis, N. Occurrence and Fate
of Ozonation By-Products at a Full-Scale Drinking Water Treatment
Plant. Sci. Total Environ. 2014, 481 (1), 392
−400.
(92) Richardson, S. D.; Plewa, M. J.; Wagner, E. D.; Schoeny, R.;
DeMarini, D. M. Occurrence, Genotoxicity, and Carcinogenicity of
Regulated and Emerging Disinfection by-Products in Drinking Water:
A Review and Roadmap for Research. Mutat. Res., Rev. Mutat. Res. 2007,
636, 178−242.
(93) Bal, R.; Tu
̈
rk, G.; Tuzcu, M.; Yilmaz, O.; Kuloglu, T.; Gundogdu,
R.; Gu
̈
r, S.; Agca, A.; Ulas, M.; Çambay, Z.; Tuzcu, Z.; Gencoglu, H.;
Guvenc, M.; Ozsahin, A. D.; Kocaman, N.; Aslan, A.; Etem, E.
Assessment of Imidacloprid Toxicity on Reproductive Organ System of
Adult Male Rats. J. Environ. Sci. Health, Part B 2012, 47 (5), 434−444.
(94) Hrudey, S. E.; Bull, R. J.; Cotruvo, J. A.; Paoli, G.; Wilson, M.
Drinking Water as a Proportion of Total Human Exposure to Volatile
N-Nitrosamines. Risk Anal. 2013, 33 (12), 2179−2208.
(95) Bei, E.; Shu, Y.; Li, S.; Liao, X.; Wang, J.; Zhang, X.; Chen, C.;
Krasner, S. Occurrence of Nitrosamines and Their Precursors in
Drinking Water Systems around Mainland China. Water Res. 2016, 98,
168−175.
(96) Farré, M. J.; Radjenovic, J.; Gernjak, W. Asses sment of
Degradation Byproducts and NDMA Formation Potential during Uv
and UV/H2o2 Treatment of Doxylamine in the Presence of
Monochloramine. Environ. Sci. Technol. 2012, 46 (23), 12904−12912.
ACS ES&T Engineering pubs.acs.org/estengg Review
https://doi.org/10.1021/acsestengg.1c00258
ACS EST Engg. XXXX, XXX, XXX−XXX
M

(97) Crone, B. C.; Speth, T. F.; Wahman, D. G.; Smith, S. J.;
Abulikemu, G.; Kleiner, E. J.; Pressman, J. G. Occurrence of Per- and
Polyfluoroalkyl Substances (PFAS) in Source Water and Their
Treatment in Drinking Water. Crit. Rev. Environ. Sci. Technol. 2019,
49 (24), 2359−2396.
(98) Domingo, J. L.; Nadal, M. Human Exposure to Per- and
Polyfluoroalkyl Substances (PFAS) through Drinking Water: A Review
of the Recent Scientific Literature. Environ. Res. 2019, 177, 108648.
(99) Maddela, N. R.; Venkateswarlu, K.; Megharaj, M. Tris(2-
Chloroethyl) Phosphate, a Pervasive Flame Retardant: Critical
Perspective on Its Emissions into the Environment and Human
Toxicity. Environ. Sci.: Processes Impacts 2020, 22, 1809−1827.
(100) Rodil, R.; Quintana, J. B.; Concha-Gran
̃
a, E.; López-Mahía, P.;
Muniategui-Lorenzo, S.; Prada-Rodríguez, D. Emerging Pollutants in
Sewage, Surface and Drinking Water in Galicia (NW Spain).
Chemosphere 2012, 86 (10), 1040−1049.
(101) Ding, J.; Shen, X.; Liu, W.; Covaci, A.; Yang, F. Occurrence and
Risk Assessment of Organophosphate Esters in Drinking Water from
Eastern China. Sci. Total Environ. 2015, 538, 959−965.
(102) Bukowski, K.; Wysokinski, D.; Mokra, K.; Wozniak, K. DNA
Damage and M ethy lati on In duced by Organophosphate Flame
Retardants: Tris(2-Chloro ethyl) Phosphate and Tris(1-Chloro-2-
Propyl) Phosphate in Human Peripheral Blood Mononuclear Cells.
Hum. Exp. Toxicol. 2019, 38 (6), 724−733.
(103) Olaniyan, L. W. B.; Mkwetshana, N.; Okoh, A. I. Triclosan in
Water, Implications for Human and Environmental Health. Spring-
erPlus 2016, 5, 1639.
(104) Salveson, A.; Dickenson, E.; Soller, J.; Angelotti, B.; Parker, A.
Pathogen Risk Evaluation of Treatment and Monitoring System Perform-
ance for Potable Reuse; Denver, CO, 2018.
(105) Fujioka, T.; Makabe, R.; Mori, N.; Snyder, S. A.; Leddy, M.
Assessment of Online Bacterial Particle Counts for Monitoring the
Performance of Reverse Osmosis Membrane Process in Potable Reuse.
Sci. Total Environ. 2019, 667, 540−544.
(106) Amoueyan, E.; Ahmad, S.; Eisenberg, J. N. S.; Pecson, B.;
Gerrity, D. Quantifying Pathogen Risks Associated with Potable Reuse:
A Risk Assessment Case Study for Cryptosporidium. Water Res. 2017,
119, 252−266.
(107) Shrestha, J.; Li, J. Influence of Permeate from Domestic Reverse
Osmosis Filters on Lead Pipes Corrosion and Plastic Pipes Leaching. J.
Water Process Eng. 2017
, 18, 126−133.
(108) Xu, X.; Liu, S.; Liu, Y.; Smith, K.; Wang, X.; Li, J.; Ma, Z.; Wang,
Z.; Cui, Y. Water Quality Induced Corrosion of Stainless Steel Valves
during Long-Term Service in a Reverse Osmosis System. J. Environ. Sci.
(Beijing, China) 2020, 89, 218−226.
(109) Negm, A.; Fouda, A. e.; Shalof, R.; Mohamed, S.; Youssif, t.
Cooling System Pipeline Corrosion Behavior after Reusing of Reverse
Osmosis Reject Plant Water as Feed Water Source and Using a New
Isatine Derivatives as Corrosion Inhibitors. Egypt. J. Chem. 2019, 62
(2), 257−280.
(110) Cipollina, A.; Micale, G.; Rizzuti, L. A Brine Evaporative
Cooler/Concentrator for Autonomous Thermal Desalination Units.
Desalin. Water Treat. 2011, 31 (1−3), 269−278.
(111) Lee, S.; Choi, J.; Park, Y. G.; Shon, H.; Ahn, C. H.; Kim, S. H.
Hybrid Desalination Processes for Beneficial Use of Reverse Osmosis
Brine: Current Status and Future Prospects. Desalination 2019, 454,
104−111.
(112) Fournier, E. D.; Keller, A. A.; Geyer, R.; Frew, J. Investigating
the Energy-Water Usage Efficiency of the Reuse of Treated Municipal
Wastewater for Artificial Groundwater Recharge. Environ. Sci. Technol.
2016, 50 (4), 2044−2053.
(113) Wang, Y.; Wang, X.; Li, M.; Dong, J.; Sun, C.; Chen, G. Removal
of Pharmace utica l and Personal Care Products ( PPC Ps) from
Municipal Waste Water with Integrated Membrane Systems, MBR-
RO/NF. Int. J. Environ. Res. Public Health 2018, 15 (2), 269.
(114) Wang, Y.; Huang, H. Carbon Nanotube Composite Membranes
for Microfiltration of Pharmaceuticals and Personal Care Products. In
Advanced Nanomaterials for Membrane Synthesis and its Applications;
Elsevier Inc., 2019; DOI: 10.1016/B978-0-12-814503-6.00008-2.
(115) Franke, V.; Ullberg, M.; McCleaf, P.; Wålinder, M.; Köhler, S. J.;
Ahrens, L. The Price of Really Clean Water: Combining Nanofiltration
with Granular Activated Carbon and Anion Exchange Resins for the
Removal of Per- And Polyfluoralkyl Substances (PFASs) in Drinking
Water Production. ACS ES&T Water 2021, 1 (4), 782−795.
(116) Yangali-Quintanilla, V.; Maeng, S. K.; Fujioka, T.; Kennedy, M.;
Li, Z.; Amy, G. Nanofiltration vs. Reverse Osmosis for the Removal of
Emerging Organic Contaminants in Water Reuse. Desalin. Water Treat.
2011, 34 (1−3), 50−56.
(117) Mastropietro, T. F.; Bruno, R.; Pardo, E.; Armentano, D.
Reverse Osmosis and Nanofiltration Membranes for Highly Efficient
PFASs Removal: Overview, Challenges and Future Perspectives. Dalt.
Trans. 2021, 50 (16), 5398−5410.
(118) Zheng, L.; Yu, D.; Wang, G.; Yue, Z.; Zhang, C.; Wang, Y.;
Zhang, J.; Wang, J.; Liang, G.; Wei, Y. Characteristics and Formation
Mechanism of Membrane Fouling in a Full-Scale RO Wastewater
Reclamation Process: Membrane Autopsy and Fouling Character-
ization. J. Membr. Sci. 2018, 563, 843−856.
(119) Jiang, S.; Li, Y.; Ladewig, B. P. A Review of Reverse Osmosis
Membrane Fouling and Control Strategies. Sci. Total Environ. 2017,
595, 567−583.
(120) Ridgway, H. F.; Orbell, J.; Gray, S. Molecular Simulations of
Polyamide Membrane Materials Used in Desalination and Water Reuse
Applications: Recent Developments and Future Prospects. J. Membr.
Sci. 2017, 524, 436−448.
(121) Tang, C. Y.; Chong, T. H.; Fane, A. G. Colloidal Interactions
and Fouling of NF and RO Membranes: A Review. Adv. Colloid Interface
Sci. 2011, 164 (1−2), 126−143.
(122) Park, J.; Jeong, K.; Baek, S.; Park, S.; Ligaray, M.; Chong, T. H.;
Cho, K. H. Modeling of NF/RO Membrane Fouling and Flux Decline
Using Real-Time Observations. J. Membr. Sci. 2019, 576,66−77.
(123) Zhao, D.; Yu, S. A Review of Recent Advance in Fouling
Mitigation of NF/RO Membranes in Water Treatment: Pretreatment,
Membrane Modification, and Chemical Cleaning. Desalin. Water Treat.
2015, 55 (4), 870−891.
(124) Tian, M.; Xu, H. J.; Yao, L.; Wang, R. A Biomimetic
Antimicrobial Surface for Membrane Fouling Control in Reverse
Osmosis for Seawater Desalination. Desalination 2021, 503, 114954.
(125) Vatanpour, V.; Safarpour, M.; Khataee, A.; Zarrabi, H.;
Yekavalangi, M. E.; Kavian, M. A Thin Film Nanocomposite Reverse
Osmosis Membrane Containing Amine-Functionalized Carbon Nano-
tubes. Sep. Purif. Technol. 2017, 184, 135−143.
(126) Cohen-Tanugi, D.; Grossman, J. C. Nanoporous Graphene as a
Reverse Osmos is Membrane: Recent Insights from Theory and
Simulation. Desalination 2015, 366
,59−70.
(127) Jafarzadeh, R.; Azamat, J.; Erfan-Niya, H.; Hosseini, M.
Molecular Insights into Effective Water Desalination through Function-
alized Nanoporous Boron Nitride Nanosheet Membranes. Appl. Surf.
Sci. 2019, 471, 921−928.
(128) Zhang, L.; Jia, L.; Zhang, J.; Li, J.; Liang, L.; Kong, Z.; Shen, J.
W.; Wang, X.; Zhang, W.; Wang, H. Understanding the Effect of
Chemical Modification on Water Desalination in Boron Nitride
Nanotubes via Molecular Dynamics Simulation. Desalination 2019,
464,84−93.
(129) Ebro, H.; Kim, Y. M.; Kim, J. H. Molecular Dynamics
Simulations in Membrane -Based Water Treatment Processes: A
Systematic Overview. J. Membr. Sci. 2013, 438, 112−125.
(130) Alsarayreh, A. A.; Al-Obaidi, M. A.; Farag, S. K.; Patel, R.;
Mujtaba, I. M. Performance Evaluation of a Medium-Scale Industrial
Reverse Osmosis Brackish Water Desalination Plant with Different
Brands of Membranes. A Simulation Study. Desalination 2021, 503,
114927.
(131) Okamoto, Y.; Lienhard, J. H. How RO Membrane Permeability
and Other Performance Factors Affect Process Cost and Energy Use: A
Review. Desalination 2019 , 470, 114064.
(132) Galizia, A.; Mamo, J.; Blandin, G.; Verdaguer, M.; Comas, J.;
Rodríguez-Roda, I.; Monclu
́
s, H. Advanced Control System for Reverse
Osmosis Optimization in Water Reuse Systems. Desalination 2021, 518,
115284.
ACS ES&T Engineering pubs.acs.org/estengg Review
https://doi.org/10.1021/acsestengg.1c00258
ACS EST Engg. XXXX, XXX, XXX−XXX
N

(133) Yu, R.-F.; Chen, H.-W.; Cheng, W.-P.; Hsieh, P.-H. Dosage
Control of the Fenton Process for Color Removal of Textile
Wastewater Applying ORP Monitoring and Artificial Neural Networks.
J. Environ. Eng. 2009, 135 (5), 325−332.
(134) Giwa, A.; Yusuf, A.; Balogun, H. A.; Sambudi, N. S.; Bilad, M. R.;
Adeyemi, I.; Chakraborty, S.; Curcio, S. Recent Advances in Advanced
Oxidation Processes for Removal of Contaminants from Water: A
Comprehensive Review. Process Saf. Environ. Prot. 2021, 146, 220−256.
(135) Aleboyeh, A.; Kasiri, M. B.; Olya, M. E.; Aleboyeh, H.
Prediction of Azo Dye Decolorization by UV/H2O2 Using Artificial
Neural Networks. Dyes Pigm. 2008, 77 (2), 288−294.
(136) do Nascimento, G. E.; Napolea
̃
o, D. C.; da Rocha Santana, R.
M.; Charamba, L. V. C.; de Oliveira, J. G. C.; de Moura, M. C.; Coelho,
L. C. B. B.; Duarte, M. M. M. B. Degradation of Textile Dyes Remazol
Yellow Gold and Reactive Turquoise: Optimization, Toxicity and
Modeling by Artificial Neural Networks. Water Sci. Technol. 2018, 2017
(3), 812−823.
(137) Zarei, M.; Niaei, A.; Salari, D.; Khataee, A. R. Removal of Four
Dyes from Aqueous Medium by the Peroxi-Coagulation Method Using
Carbon Nanotube-PTFE Cathode and Neural Network Modeling. J.
Electroanal. Chem. 2010, 639 (1−2), 167−174.
(138) Zarei, M.; Khataee, A. R.; Ordikhani-Seyedlar, R.; Fathinia, M.
Photoelectro-Fenton Combined with Photocatalytic Process for
Degradation of an Azo Dye Using Supported TiO2 Nanoparticles
and Carbon Nanotube Cathode: Neural Network Modeling. Electro-
chim. Acta 2010, 55 (24), 7259−7265.
(139) Jing, L.; Chen, B.; Wen, D.; Zheng, J.; Zhang, B. Pilot-Scale
Treatment of Atrazine Production Wastewater by UV/O3/Ultrasound:
Factor Effects and System Optimization. J. Environ. Manage. 2017, 203,
182−190.
(140) Vaferi, B.; Bahmani, M.; Keshavarz, P.; Mowla, D. Experimental
and Theoretical Analysis of the UV/H2O2 Advanced Oxidation
Processes Treating Aromatic Hydrocar bons and MTBE from
Contaminated Synthetic Wastewaters. J. Environ. Chem. Eng. 2014, 2
(3), 1252−1260.
(141) Amani-Ghadim, A. R.; Dorraji, M. S. S. Modeling of
Photocatalyatic Process on Synthesized ZnO Nanoparticles: Kinetic
Model Development and Artificial Neural Networks. Appl. Catal., B
2015, 163 , 539−546.
(142) Liang, C. H.; Chiang, P. C.; Chang, E. E. Modeling the
Behaviors of Adsorption and Biodegradation in Biological Activated
Carbon Filters. Water Res. 2007, 41 (15), 3241−3250.
(143) Zhiteneva, V.; Ziemendorf, E
́
.; Sperlich, A.; Drewes, J. E.;
Hu
̈
bner, U. Differentiating between Adsorption and Biodegradation
Mechanisms While Removing Trace Organic Chemicals (TOrCs) in
Biological Activated Carbon (BAC) Filters. Sci. Total Environ. 2020,
743, 140567.
(144) Gerrity, D.; Owens-Bennett, E.; Venezia, T.; Stanford, B. D.;
Plumlee, M. H.; Debroux, J.; Trussell, R. S. Applicability of Ozone and
Biological Activated Carbon for Potable Reuse. Ozone: Sci. Eng. 2014,
36 (2), 123−137.
(145) Tang, C. Y.; Yang, Z.; Guo, H.; Wen, J. J.; Nghiem, L. D.;
Cornelissen, E. Potable Water Reuse through Advanced Membrane
Technology. Environ. Sci. Technol. 2018, 52 (18), 10215−10223.
(146) Ozaki, H.; Sharma, K.; Saktaywin, W. Performance of an Ultra-
Low-Pressure Reverse Osmosis Membrane (ULPROM) for Separating
Heavy Metal: Effects of Interference Parameters. Desalination 2002,
144 (1−3), 287−294.
(147) Víctor-Ortega, M. D.; Ratnaweera, H. C. Double Filtration as an
Effective System for Removal of Arsenate and Arsenite from Drinking
Water through Reverse Osmosis. Process Saf. Environ. Prot. 2017, 111,
399−408.
(148) Abejón, A.; Garea, A.; Irabien, A. Arsenic Removal from
Drinking Water by Reverse Osmosis: Minimization of Costs and
Energy Consumption. Sep. Purif. Technol. 2015, 144,46−53.
(149) Bernstein, R.; Belfer, S.; Freger, V. Toward Improved Boron
Removal in RO by Membrane Modification: Feasibility and Challenges.
Environ. Sci. Technol. 2011, 45 (8), 3613−3620.
(150) Jung, B.; Kim, C. Y.; Jiao, S.; Rao, U.; Dudchenko, A. V.; Tester,
J.; Jassby, D. Enhancing Boron Rejection on Electrically Conducting
Reverse Osmosis Membranes through Local Electrochemical PH
Modification. Desalination 2020, 476, 114212.
(151) Mel’nik, L. A.; Babak, Y. V.; Goncharuk, V. V. Problems of
Removing Arsenic Compounds from Natural Water in the Pressure
Driven Treatment Process. J. Water Chem. Technol. 2012, 34 (3), 162−
167.
(152) Uddin, T.; Mozumder, S. I.; Figoli, A.; Islam, A.; Drioli, E.
Arsenic Removal by Conventional and Membrane Technology: An
Overview. Indian J. Chem. Technol. 2007, 14, 441.
(153) Kabay, N.; Gu
̈
ler, E.; Bryjak, M. Boron in Seawater and
Methods for Its Separation - A Review. Desalination 2010, 261, 212
−
217.
(154) Zhu, X.; Jassby, D. Electroactive Membranes for Wate r
Treatment: Enhanced Treatment Functionalities, Energy Consider-
ations, and Future Challenges. Acc. Chem. Res. 2019, 52 (5), 1177−
1186.
(155) Dorji, P.; Choi, J.; Kim, D. I.; Phuntsho, S.; Hong, S.; Shon, H.
K. Membrane Capacitive Deionisation as an Alternative to the 2nd Pass
for Seawater Rev erse Osmosis Desalinatio n Plant f or Brom ide
Removal. Desalination 2018, 433, 113−119.
(156) Breitner, L. N.; Howe, K. J.; Minakata, D. Effect of Functional
Chemistry on the Rejecti on of Low-Molecular Weight Neutral
Organics through Reverse Osmosis Membranes for Potable Reuse.
Environ. Sci. Technol. 2019, 53 (19), 11401−11409.
(157) Dorji, P.; Inhyuk, D.; Hong, S.; Phuntsho, S.; Kyong, H. Pilot-
Scale Membrane Capacitive Deionisation for e Ff Ective Bromide
Removal and High Water Recovery in Seawater Desalination.
Desalination 2020, 479, 114309.
(158) Soyluoglu, M.; Ersan, M. S.; Ateia, M.; Karanfil, T. Removal of
Bromide from Natural Waters: Bromide-Selective vs. Conventional Ion
Exchange Resins. Chemosphere 2020, 238, 124583.
(159) Govind, R.; Lai, L.; Dobbs, R. Integrated Model for Predicting
the Fate of Organics in Wastewater Treatment Plants. Environ. Prog.
1991, 10 (1), 13−23.
(160) Arsuaga, J. M.; Sotto, A.; López-Mun
̃
oz, M. J.; Braeken, L.
Influence of Type and Position of Functional Groups of Phenolic
Compounds on NF/RO Performance. J. Membr. Sci. 2011, 372 (1−2),
380−386.
(161) Cheng, H.; Reinhard, M. Sorption of Trichloroethylene in
Hydrophobic Micropores of Dealuminated Y Zeolites and Natural
Minerals. Environ. Sci. Technol. 2006, 40 (24), 7694−7701.
(162) Abdelbassit, M. S.; Popoola, S. A.; Saleh, T. A.; Abdallah, H. H.;
Al-Saadi, A. A.; Alhooshani, K. R. DFT and Kinetic Evaluation of
Chloromethane Removal Using Cost-Effective Activated Carbon.
Arabian J. Sci. Eng. 2020, 45 (6), 4705−4716.
(163) Valdés, H.; Riquelme, A. L.; Solar, V. A.; Azzolina-Jury, F.;
Thibault-Starzyk, F. Removal of C hlorinated Volatile Organic
Compounds onto Natural and Cu-Modified Zeolite: The Role of
Chemical Surface Characteristics in the Adsorption Mechanism. Sep.
Purif. Technol. 2021, 258, 118080.
(164) Ece, M. Ş.; Kutluay, S.; Şahin, O
̈
.; Horoz, S. Development of
Novel Fe3O4/AC@SiO2@1,4-DAAQ Magnetic Nanoparticles with
Outstanding VOC Removal Capacity: Characterization, Optimization,
Reusability, Kinetics, and Equilibrium Studies. Ind. Eng. Chem. Res.
2020
, 59 (48), 21106−21123.
(165) Adeleye, A. S.; Conway, J. R.; Garner, K.; Huang, Y.; Su, Y.;
Keller, A. A. Engineered Nanomaterials for Water Treatment and
Remediation: Costs, Benefits, and Applicability. Chem. Eng. J. 2016,
286, 640−662.
(166) Narayanan, B.; Suidan, M. T.; Gelderloos, A. B.; Brenner, R. C.
Anaerobic Treatment of Volatile and Semivolatile Organic Compounds
in Municipal Wastewater. Water Environ. Res. 1995, 67, 46.
(167) Wu, W.; Shi, J.; Hickey, R. F. Long-Term Performance of Co-
Metabolic Degradation of Trichloroethylene in a Fluidized Bed Reactor
Fed with Benzene, Toluene and Xylene. J. Chem. Technol. Biotechnol.
2008, 83, 513− 523.
ACS ES&T Engineering pubs.acs.org/estengg Review
https://doi.org/10.1021/acsestengg.1c00258
ACS EST Engg. XXXX, XXX, XXX−XXX
O

(168) Jans, U.; Hoigné, J. Activated Carbon and Carbon Black
Catalyzed Transformation of Aqueous Ozone into OH-Radicals.
Ozone: Sci. Eng. 1998, 20 (1), 67−90.
(169) Sánchez-Polo, M.; Salhi, E.; Rivera-Utrilla, J.; Von Gunten, U.
Combination of Ozone with Activated Carbon as an Alternative to
Conventional Advanced Oxidation Processes. Ozone: Sci. Eng. 2006, 28
(4), 237−245.
(170) Marghade, D.; Vaidya, A.; Yenkie, M. K. N.; Pokale, W. K.
Feasibility Study of UV Assisted Different Heterogenous Photo-
Catalytic Methods for Degradation of 2-Nitrophenol in Aqueous Phase.
Mater. Today Proc. 2020, 29, 1166−1170.
(171) Gerrity, D.; Snyder, S. Review of Ozone for Water Reuse
Applications: Toxicity, Regulations, and Trace Organic Contaminant
Oxidation. Ozone: Sci. Eng. 2011, 33 (4), 253−266.
(172) Salimi, M.; Esrafili, A.; Gholami, M.; Jonidi Jafari, A.; Rezaei
Kalantary, R.; Farzadkia, M.; Kermani, M.; Sobhi, H. R. Contaminants
of Emerging Concern: A Review of New Approach in AOP
Technologies. Environ. Monit. Assess. 2017, 189 (8), 1−22.
(173) Oneby, M. A.; Bromley, C. O.; Borchardt, J. H.; Harrison, D. S.
Ozone Treatment of Secondary Effluent at U.S. Municipal Wastewater
Treatment Plants. Ozone: Sci. Eng. 2010, 32 (1), 43−55.
(174) Rodriguez, E.; Campinas, M.; Acero, J. L.; Rosa, M. J.
Investigating PPCP Removal from Wastewater by Powdered Activated
Carbon/Ultrafiltration. Water, Air, Soil Pollut. 2016, 227 (6), 177.
(175) McCleaf, P.; Englund, S.; O
̈
stlund, A.; Lindegren, K.; Wiberg,
K.; Ahrens, L. Removal Efficiency of Multiple Poly- and Perfluoroalkyl
Substances (PFASs) in Drinking Water Using Granular Activated
Carbon (GAC) and Anion Exchange (AE) Column Tests. Water Res.
2017, 120 ,77−87.
(176) Zhang, Y.; Ozcer, P.; Ghoshal, S. A Comprehensive Assessment
of the Degradation of C1 and C2 Chlorinated Hydrocarbons by
Sulfidated Nanoscale Zerovalent Iron. Water Res. 2021, 201 (March),
117328.
(177) Liu, Z.; Betterton, E. A.; Arnold, R. G. Electrolytic Reduction of
Low Molecular Weight Chlorinated Aliphatic Compounds: Structural
and Thermodynamic Effects on Process Kinetics. Environ. Sci. Technol.
2000, 34 (5), 804−811.
(178) Fernández-Verdejo, D.; Sulonen, L. K.; Pérez-Trujillo, M.;
Marco-Urrea, E.; Blánquez, P. Electrochemical Dehalogenation of to
Non-Toxic Products Using a Carbon Fiber Brush Electrode. J. Chem.
Technol. Biotechnol. 2021, 96
(2), 335−340.
(179) Iervolino, G.; Zammit, I.; Vaiano, V.; Rizzo, L. Limitations and
Prospects for Wastewater Treatment by UV and Visible-Light-Active
Heterogeneous Photocatalysis: A Critical Review. Top. Curr. Chem.
2020, 378 (1), 225−264.
(180) Hodges, B. C.; Cates, E. L.; Kim, J. H. Challenges and Prospects
of Advanced Oxidation Water Treatment Processes Using Catalytic
Nanomaterials. Nat. Nanotechnol. 2018, 13 (8), 642−650.
(181) Su, Y.; Rao, U.; Khor, C. M.; Jensen, M. G.; Teesch, L. M.;
Wong, B. M.; Cwiertny, D. M.; Jassby, D. Potential-Driven Electron
Transfer Lowers the Dissociation Energy of the C - F Bond and
Facilitates Reductive Defluorination of Perfluorooctane Sulfonate
(PFOS). ACS Appl. Mater. Interfaces 2019, 11 (37), 33913−33922.
(182) Urtiaga, A. M.; Pérez, G.; Ibán
̃
ez, R.; Ortiz, I. Removal of
Pharmaceuticals from a WWTP Secondary Effluent by Ultrafiltration/
Reverse Osmosis Followed by Electrochemical Oxidation of the RO
Concentrate. Desalination 2013, 331,26−34.
(183) Krauss, M.; Longree, P.; Dorusch, F.; Ort, C.; Hollender, J.
Occurrence and Removal of N -Nitrosamines in Wastewater Treatment
Plants. Water Res. 2009, 43, 4381−4391.
(184) Yoon, S.; Nakada, N.; Tanaka, H. Occurrence and Fate of N
-Nitrosamines and Their Formation Potential in Three Wastewater
Treatment Plants in Japan Suchul Yoon, Norihide Nakada and Hiroaki
Tanaka. Water Sci. Technol. 2013, 68 (10), 2118−2127.
(185) Asami, M.; Oya, M.; Kosaka, K. A Nationwide Survey of NDMA
in Raw and Drinking Water in Japan. Sci. Total Environ. 2009, 407 (11),
3540−3545.
(186) Yoon, S.; Nakada, N.; Tanaka, H. Occurrence and Removal of
NDMA and NDMA Formation Potential in Wastewater Treatment
Plants. J. Hazard. Mater. 2011, 190 (1−3), 897−902.
(187) Padhye, L.; Tezel, U.; Mitch, W. A. Occurrence and Fate of
Nitrosamines and Their Precursors in Municipal Sludge and Anaerobic
Digestion Systems. Environ. Sci. Technol. 2009, 43 (9), 3087−3093.
(188) Krasner, S. W.; Westerhoff, P.; Mitch, W. A.; Hanigan, D.;
McCurry, D. L.; Von Gunten, U. Behavior of NDMA Precursors at 21
Full-Scale Water Treatment Facilities. Environ. Sci. Water Res. Technol.
2018, 4 (12), 1966
−1978.
(189) Ersan, M. S.; Ladner, D. A.; Karanfil, T. N-Nitrosodimethyl-
amine (NDMA) Precursors Leach from Nanofiltration Membranes.
Environ. Sci. Technol. Lett. 2015, 2 (3), 66−69.
(190) Fujioka, T.; Khan, S. J.; McDonald, J. A.; Roux, A.; Poussade, Y.;
Drewes, J. E.; Nghiem, L. D. N-Nitrosamine Rejection by Nano-
filtration and Reverse Osmosis Membranes: The Importance of
Membrane Characteristics. Desalination 2013, 316,67−75.
(191) Mitch, W. A.; Sharp, J. O.; Trussell, R. R.; Valentine, R. L.;
Alvarez-Cohen, L.; Sedlak, D. L. N-Nitrosodimethylamine (NDMA) as
a Drinking Water Contaminant: A Review. Environ. Eng. Sci. 2003,
389−404.
(192) Mitch, W. A.; Sharp, J. O.; Trussell, R. R.; Valentine, R. L.;
Alvarez-Cohen, L.; Sedlak, D. L. N -Nitrosodimethylamine (NDMA) as
a Drinking Water Contaminant: A Review. Environ. Eng. Sci. 2003, 20
(5), 389−404.
(193) Fujioka, T.; Osako, M.; Tanabe, S.; Kodamatani, H.; Shintani,
T. Plugging Nonporous Polyamide Membranes for Enhance d
Rejection of Small Contaminants during Advanced Wastewater
Treatment. Sep. Purif. Technol. 2020, 253, 117490.
(194) Croll, H.; Soroush, A.; Pillsbury, M. E.; Romero-Vargas
Castrillón, S. Graphene Oxide Surface Modification of Polyamide
Reverse Osmosis Membranes for Improved N-Nitrosodimethylamine
(NDMA) Removal. Sep. Purif. Technol. 2019, 210, 973−980.
(195) Roback, S. L.; Ishida, K. P.; Chuang, Y.-H.; Zhang, Z.; Mitch, W.
A.; Plumlee, M. H. Pilot UV-AOP Comparison of UV/Hydrogen
Peroxide, UV/Free Chl orine, and UV/Monochloramine for the
Removal of N -Nitrosodimethylamine (NDMA) and NDMA
Precursors. ACS ES&T Water 2021, 1 (2), 396−406.
(196) Fujioka, T.; Ishida, K. P.; Shintani, T.; Kodamatani, H. High
Rejection Reverse Osmosis Membrane for Removal of N - Nitros-
amines and Their Precursors. Water Res. 2018, 131,45−51.
(197) Roback, S. L.; Ishida, K. P.; Plumlee, M. H. Chemosphere In Fl
Uence of Reverse Osmosis Membrane Age on Rejection of NDMA
Precursors and Formation of NDMA in Fi Nished Water after Full
Advanced Treatment for Potable Reuse. Chemosphere 2019, 233, 120−
131.
(198) Pisarenko, A. N.; Stanford, B. D.; Yan, D.; Gerrity, D.; Snyder, S.
A. Effects of Ozone and Ozone/Peroxide on Trace Organic
Contaminants and NDMA in Drinking Water and Water Reuse
Applications. Water Res. 2012, 46 (2), 316−326.
(199) Kommineni, S.; Ela, W. P.; Arnold, R. G.; Huling, S. G.; Hester,
B. J.; Betterton, E. A. NDMA Treatment by Sequential GAC
Adsorption and Fenton-Driven Destruction. Environ. Eng. Sci. 2003,
20 (4), 361−373.
(200) Fujioka, T.; Kodamatani, H.; Takeuchi, H.; Tanaka, H.;
Nghiem, L. D. Online Monitoring of: N -Nitrosodimethylamine for the
Removal Assurance of 1,4-Dioxane and Other Trace Organic
Compounds by Reverse Osmosis. Environ. Sci. Water Res. Technol.
2018, 4 (12), 2021−2028.
(201) Schoonenberg Kegel, F.; Rietman, B. M.; Verliefde, A. R. D.
Reverse Osmosis Followed by Activated Carbon Filtration for Efficient
Removal of Organic Micropollutants from River Bank Filtrate. Water
Sci. Technol. 2010, 61 (10), 2603−2610.
(202) Andaluri, G.; Suri, R. Removal of 1,4-Dioxane and Volatile
Organic Compounds from Groundwater Using Ozone-Based Ad-
vanced Oxidation Process. Ozone: Sci. Eng. 2017, 39 (6), 423−434.
(203) Weng, C.; Chuang, Y. H.; Davey, B.; Mitch, W. A. Reductive
Electrochemical Activation of Hydrogen Peroxide as an Advanced
ACS ES&T Engineering pubs.acs.org/estengg Review
https://doi.org/10.1021/acsestengg.1c00258
ACS EST Engg. XXXX, XXX, XXX−XXX
P

Oxidation Process for Treatment of Reverse Osmosis Permeate during
Potable Reuse. Environ. Sci. Technol. 2020, 54 (19), 12593−12601.
(204) Vatankhah, H.; Szczuka, A.; Mitch, W. A.; Almaraz, N.;
Brannum, J.; Bellona, C. Evaluation of Enhanced Ozone-Biologically
Active Filtration Treatment for the Removal of 1,4-Dioxane and
Disinfection Byproduct Precursors from Wastewater Effluent. Environ.
Sci. Technol. 2019, 53 (5), 2720−2730.
(205) Myers, M. A.; Johnson, N. W.; Marin, E. Z.; Pornwongthong, P.;
Liu, Y.; Gedalanga, P. B.; Mahendra, S. Abiotic and Bioaugmented
Granular Activated Carbon for the Treatment of 1,4-Dioxane-
Contaminated Water. Environ. Pollut. 2018, 240, 916−924.
(206) Duan, D. From the Smallest Virus to the Biggest Gene:
Marching Towards Gene Therapy for Duchenne Muscular Dystrophy.
Discovery Med. 2006, 6 (33), 103−108.
(207) Bennett, A. Drinking Water: Pathogen Removal from Water -
Technologies and Techniques. Filtr. Sep. 2008, 45 (10), 14−16.
(208) Sirivithayapakorn, S.; Keller, A. Transport of Colloids in
Saturated Porous Media: A Pore-Scale Observation of the Size
Exclusion Effect and Colloid Acceleration. Water Resour. Res. 2003,
39,1.
(209) Adham, S. S.; Trussell, R. S.; Gagliardo, P. F.; Trussell, R. R.
Rejection of MS-2 Virus by RO Membranes. J. - Am. Water Works Assoc.
1998, 90 (9), 130−135.
(210) Szczuka, A.; Chuang , Y.-H.; Chen, F. C.; Zhang, Z.;
Desormeaux, E.; Flynn, M.; Parodi, J.; Mitch, W. A. Removal of
Pathogens and Chemicals of Emerging Concern by Pilot-Scale FO-RO
Hybrid Units Treating RO Concentrate, Graywater, and Sewage for
Centralized and Decentralized Potable Reuse. ACS ES&T Water 2021,
1 (1), 89−100.
(211) Antony, A.; Blackbeard, J.; Leslie, G. Removal Efficiency and
Integrity Monitoring Techniques for Virus Removal by Membrane
Processes. Crit. Rev. Environ. Sci. Technol. 2012, 42 (9), 891− 933.
(212) Pype, M. L.; Lawrence, M. G.; Keller, J.; Gernjak, W. Reverse
Osmosis Integrity Monitoring in Water Reuse: The Challenge to Verify
Virus Removal - A Review. Water Res. 2016, 98, 384−395.
(213) Zhang, D.; Ling, H.; Huang, X.; Li, J.; Li, W.; Yi, C.; Zhang, T.;
Jiang, Y.; He, Y.; Deng, S.; Zhang, X.; Wang, X.; Liu, Y.; Li, G.; Qu, J.
Potential Spreading Risks and Disinfection Challenges of Medical
Wastewater by the Presence of Severe Acute Respiratory Syndrome
Coronavirus 2 (SARS-CoV-2) Viral RNA in Septic Tanks of Fangcang
Hospital. Sci. Total Environ. 2020, 741, 140445.
(214) Diaz-Elsayed, N.; Rezaei, N.; Guo, T.; Mohebbi, S.; Zhang, Q.
Wastewater-Based Resource Recovery Technologies across Scale: A
Review. Resources, Conservation and Recycling
2019, 145 ,94−112.
(215) Hernández-Chover, V.; Bellver-Domingo, A
́
.; Hernández-
Sancho, F. Efficiency of Waste water Treat ment F aciliti es: The
Influence of Scale Economies. J. Environ. Manage. 2018, 228,77−84.
(216) Worthington, A. C.; Higgs, H. Economies of Scale and Scope in
Australian Urban Water Utilities. Util. Policy 2014, 31,52−62.
(217) Guo, T.; Englehardt, J. D. Principles for Scaling of Distributed
Direct Potable Water Reuse Systems: A Modeling Study. Water Res.
2015, 75, 146.
(218) Plumlee, M. H.; Stanford, B. D.; Debroux, J. F.; Hopkins, D. C.;
Snyder, S. A. Costs of Advanced Treatment in Water Reclamation.
Ozone: Sci. Eng. 2014, 36 (5), 485−495.
(219) Sim, A.; Mauter, M. S. Cost and Energy Intensity of U.S. Potable
Water Reuse Systems. Environ. Sci. Water Res. Technol. 2021, 7 (4),
748−761.
(220) Mendret, J.; Azais, A.; Favier, T.; B rosillon, S. Urban
Wastewater Reuse Using a Coupling between Nanofiltration and
Ozonation: Techno-Economic Assessment. Chem. Eng. Res. Des. 2019,
145,19−28.
(221) Margot, J.; Kienle, C.; Magnet, A.; Weil, M.; Rossi, L.; de
Alencastro, L. F.; Abegglen, C.; Thonney, D.; Che
̀
vre, N.; Schärer, M.;
Barry, D. A. Treatment of Micropollutants in Municipal Wastewater:
Ozone or Powdered Activated Carbon? Sci. Total Environ. 2013, 461−
462, 480−498.
(222) Mitani, M. M. M.; Keller, A. A. A.; Sandall, O. C. C.; Rinker, R.
G. G. Mass Transfer of Ozone Using a Microporous Diffuser Reactor
System. Ozone: Sci. Eng. 2005, 27 (1), 45−51.
(223) Jafari, M.; Vanoppen, M.; van Agtmaal, J. M. C.; Cornelissen, E.
R.; Vrouwenvelder, J. S.; Verliefde, A.; van Loosdrecht, M. C. M.;
Picioreanu, C. Cost of Fouling in Full-Scale Reverse Osmosis and
Nanofiltration Installations in the Netherlands. Desalination 2021, 500,
114865.
(224) Snyder, S. A.; von Gunten, U.; Amy, G.; Debroux, J. F. Use of
Ozone in Water Reclamation for Contaminant Oxidation; Report No.
WRF-08-05; Alexandria, VA, 2014.
(225) Loutatidou, S.; Chalermthai, B.; Marpu, P. R.; Arafat, H. A.
Capital Cost Estimation of RO Plants: GCC Countries versus Southern
Europe. Desalination 2014
, 347, 103−111.
(226) Caldera, U.; Breyer, C. Learning Curve for Seawater Reverse
Osmosis Desalination Plants: Capital Cost Trend of the Past, Present,
and Future. Water Resour. Res. 2017, 53 (12), 10523−10538.
(227) Lanjewar, S.; Mukherjee, A.; Khandewal, P.; Ghosh, A. K.;
Mullick, A.; Moulik, S.; Roy, A. Thermodynamics of Synthesis and
Separation Performance of Interfacially Polymerized “Loose” Reverse
Osmosis Membrane: Benchmarking for Greywater Treatment. Chem.
Eng. J. 2021, 417, 127929.
(228) Elimelech, M.; Phillip, W. A. The Future of Seawater
Desalination: Energy, Technology, and the Environment. Science
(Washington, DC, U. S.) 2011, 333 (6043), 712−717.
(229) Mayor, B. Unraveling the Historical Economies of Scale and
Learning Effects for Desalination Technologies. Water Resour. Res.
2020, 56 (2), No. e2019WR025841.
(230) Bolton, J. R.; Bircher, K. G.; Tumas, W.; Tolman, C. A. Figures-
of-Merit for the Technical Development and Application of Advanced
Oxidation Technologies for Both Electric- and Solar-Driven Systems.
Pure Appl. Chem. 2001, 73 (4), 627−637.
(231) Miklos, D. B.; Remy, C.; Jekel, M.; Linden, K. G.; Drewes, J. E.;
Hu
̈
bner, U. Evaluation of Advanced Oxidation Processes for Water and
Wastewater Treatment - A Critical Review. Water Res. 2018, 139, 118−
131.
(232) Farkas, J.; Náfrádi, M.; Hlogyik, T.; Cora Pravda, B.; Schrantz,
K.; Hernádi, K.; Alapi, T. Comparison of Advanced Oxidation
Processes in the Decomposition of Diuron and Monuron-Efficiency,
Intermediates, Electrical Energy per Order and the Effect of Various
Matrices. Environ. Sci. Water Res. Technol. 2018, 4 (9), 1345−1360.
(233) Sanches, S.; Barreto Crespo, M. T.; Pereira, V. J. Drinking Water
Treatment of Priority Pesticides Using Low Pressure UV Photolysis
and Advanced Oxidation Processes. Water Res. 2010, 44 (6), 1809−
1818.
(234) Shu, Z.; Bolton, J. R.; Belosevic, M.; Gamal El Din, M.
Photodegradation of Emerging Micropollutants Using the Medium-
Pressure UV/H2O2 Advanced Oxidation Process. Water Res. 2013, 47
(8), 2881−2889.
(235) Ikehata, K.; El-Din, M. G.; Snyder, S. A. Ozonation and
Advanced Oxidation Treatment of Emerging Organic Pollutants in
Water and Wastewater. Ozone: Sci. Eng. 2008, 30,21−26.
(236) Benitez, F. J.; Acero, J. L.; Real, F. J.; Roldan, G.; Casas, F.
Comparison of Different Chemical Oxidation Treatments for the
Removal of Selected Pharmaceuticals in Water Matrices. Chem. Eng. J.
2011, 168 (3), 1149−1156.
(237) Huber, M. M.; Göbel, A.; Joss, A.; Hermann, N.; Löffler, D.;
McArdell, C. S.; Ried, A.; Siegrist, H.; Ternes, T. A.; Von Gunten, U.
Oxidation of Pharmaceuticals during Ozonation of Municipal Waste-
water Effluents: A Pilot Study. Environ. Sci. Technol. 2005, 39 (11),
4290−4299.
(238) Swaim, P.; Royce, A.; Smith, T.; Maloney, T.; Ehlen, D.; Carter,
B. Effectiveness of UV Advanced Oxidation for Destruction of Micro-
Pollutants. Ozone: Sci. Eng. 2008, 30,34−42.
(239) Wardenier, N.; Liu, Z.; Nikiforov, A.; Van Hulle, S. W. H.; Leys,
C. Micropollutant Elimination by O3, UV and Plasma-Based AOPs: An
Evaluation of Treatment and Energy Costs. Chemosphere 2019, 234,
715−724.
ACS ES&T Engineering pubs.acs.org/estengg Review
https://doi.org/10.1021/acsestengg.1c00258
ACS EST Engg. XXXX, XXX, XXX−XXX
Q

(240) Gingerich, D. B.; Mauter, M. S. Air Emissions Damages from
Municipal Drinking Water Treatment under Current and Proposed
Regulatory Standards. Environ. Sci. Technol. 2017, 51 (18), 10299−
10306.
(241) Keller, A. A.; Tellinghuisen, S.; Lee, C.; Larson, D.; Dennen, B.;
Lee, J. Projection of California’s Future Freshwater Requirements for
Power Generation. Energy Environ. 2010, 21 (2), 1−20.
(242) King, C. W.; Holman, A. S.; Webber, M. E. Thirst for Energy.
Nature Geoscience 2008, 1, 283−286.
(243) Plappally, A. K.; Lienhard, V. J. H. Energy Requirements for
Water Production, Treatment, End Use, Reclamation, and Disposal.
Renewable Sustainable Energy Rev. 2012, 16, 4818−4848.
(244) Liu, Y.; Mauter, M. S. Assessing the Demand Response Capacity
of U.S. Drinking Water Treatment Plants. Appl. Energy 2020, 267,
114899.
(245) Olivieri, A. W.; Crook, J.; Anderson, M. A.; Bull, R. J.; Drewes, J.
E.; Haas, C. N.; Jakubowski, W.; McCarty, P. L.; Nelson, K. L.; Rose, J.
B.; Sedlak, D. L.; Wade, T. J. Expert Panel Final Report: Evaluation of the
Feasibility of Developing Uniform Water Recycling Criteria for Direct
Potable Reuse; National Water Research Institute: Fountain Valley, CA,
2016.
(246) Etchepare, R.; van der Hoek, J. P. Health Risk Assessment of
Organic Micropollutants in Greywater for Potable Reuse. Water Res.
2015, 72, 186−198.
(247) Dewettinck, T.; Van Houtte, E.; Geenens, D.; Van Hege, K.;
Verstraete, W. HACCP (Hazard Analysis and Critical Control Points)
to Guarantee Safe Water Reuse and Drinking Water Production - A
Case Study. Water Sci. Technol. 2001, 43,31−38.
(248) Roccaro, P. Treatment Processes for Municipal Wastewater
Reclamation: The Challenges of Emerging Contaminants and Direct
Potable Reuse. Curr. Opin. Environ. Sci. Health 2018, 2,46−54.
(249) Jolis, D.; Hirano, R.; Pitt, P. Tertiary Treatment Using
Microfiltration and UV Disinfection for Water Reclamation. Water
Environ. Res. 1999, 71 (2), 224−231.
(250) Gabelich, C. J.; Yun, T. I.; Coffey, B. M.; Suffet, I. H. Pilot-Scale
Testing of Reverse Osmosis Using Conventional Treatment and
Microfiltration. Desalination 2003, 154
(3), 207−223.
(251) O’Connell, P. M.; Winters, M. Making a Case for Membrane
Replacement. Opflow 2019, 45 (2), 18−22.
(252) Adham, S.; Gagliardo, P.; Smith, D.; Ross, D.; Gramith, K.;
Trussell, R. Monitoring the Integrity of Reverse Osmosis Membranes.
Desalination 1998, 119 (1−3), 143−150.
(253) Kumar, M.; Adham, S.; DeCarolis, J. Reverse Osmosis Integrity
Monitoring. Desalination 2007, 214 (1−3), 138−149.
(254) Pype, M. L.; Patureau, D.; Wery, N.; Poussade, Y.; Gernjak, W.
Monitoring Reverse Osmosis Performance: Conductivi ty ver sus
Fluorescence Excitation-Emission Matrix (EEM). J. Membr. Sci. 2013,
428, 205−211.
(255) Naughton, D. P.; Grootveld, M.; Blake, D. R.; Guestrin, H. R.;
Narayanaswamy, R. An Optical Hydroxyl Radical Sensor. Biosens.
Bioelectron. 1993, 8 (6), 325−329.
(256) Anderson, T. N.; Lucht, R. P.; Meyer, T. R.; Roy, S.; Gord, J. R.
Diode-Laser-Based Ultraviolet-A bsorption Sensor for High-Speed
Detection of the Hydroxyl Radical. Opt. Lett. 2005, 30 (11), 1321.
(257) Modrzejewska, B.; Guwy, A. J.; Dinsdale, R.; Hawkes, D. L.
Measurement of Hydrogen Peroxide in an Advanced Oxidation Process
Using an Automated Biosensor. Water Res. 2007, 41 (1), 260−268.
(258) Song, Z. M.; Xu, Y. L.; Liang, J. K.; Peng, L.; Zhang, X. Y.; Du,
Y.; Lu, Y.; Li, X. Z.; Wu, Q. Y.; Guan, Y. T. Surrogates for On-Line
Monitoring of the Attenuation of Trace Organic Contaminants during
Advanced Oxidation Processes for Water Reuse. Water Res. 2021, 190,
116733.
(259) Drewes, J. E.; Anderson, P.; Denslow, N.; Jakubowski, W.;
Olivieri, A.; Schlenk, D.; Snyder, S. Monitoring Strategies for Constituents
of Emerging Concern (CECs) in Recycled Water; Southern California
Coastal Water Research Project: Costa Mesa, CA, 2018.
(260) Li, J.; Zhu, Y.; Wu, X.; Hoffmann, M. R. Rapid Detection
Methods for Bacterial Pathogens in Ambient Waters at the Point of
Sample Collection: A Brief Review. Clinical Infectious Diseases 2020, 71,
S84−S90.
(261) Shamsipur, M.; Jalali, F.; Ershad, S. Preparation of a Diclofenac
Potentiometric Sensor and Its Application to Pharmaceutical Analysis
and to Drug Recovery from Biological Fluids. Journal of Pharmaceutical
and Biomedical Analysis 2005, 37, 943−947.
(262) Nagaraj, V. J.; Jacobs, M.; Vattipalli, K. M.; Annam, V. P.;
Prasad, S. Nanochannel-Based Electrochemical Sensor for the
Detection of Pharmaceutical Contaminants in Water. Environ. Sci.
Process. Impacts 2014, 16 (1), 135−140.
(263) Wasswa, J.; Mladenov, N.; Pearce, W. Assessing the Potential of
Fluorescence Spectroscopy to Monitor Contaminants in Source Waters
and Water Reuse Systems. Environ. Sci. Water Res. Technol. 2019, 5 (2),
370−382.
(264) Zulkifli, S. N.; Rahim, H. A.; Lau, W. J. Detection of
Contaminants in Water Supply: A Review on State-of-the-Art
Monitoring Technologies and Their Applications. Sens. Actuators, B
2018, 255, 2657−2689.
(265) Mulvihill, M.; Tao, A.; Benjauthrit, K.; Arnold, J.; Yang, P.
Surface-Enhanced Raman Spectroscopy for Trace Arsenic Detection in
Contaminated Water. Angew. Chem., Int. Ed. 2008, 47 (34), 6456−
6460.
(266) Garciá-Miranda Ferrari, A.; Carrington, P.; Rowley-Neale, S. J.;
Banks, C. E. Recent Advances in Portable Heavy Metal Electrochemical
Sensing Platforms. Environ. Sci.: Water Res. 2020, 6, 2676−2690.
(267) Sedki, M.; Zhao, G.; Ma, S.; Jassby, D.; Mulchandani, A. Linker-
Free Magnetite-Decorated Gold Nanoparticles (Fe3 O4-Au): Syn-
thesis, Characterization, and Application for Electrochemical Detection
of Arsenic (III). Sensors 2021, 21 (3), 883.
(268) Buledi, J. A.; Amin, S.; Haider, S. I.; Bhanger, M. I.; Solangi, A.
R. A Review on Detection of Heavy Metals from Aqueous Media Using
Nanomaterial-Based Sensors. Environ. Sci. Pollut. Res. 2020,1−9.
(269) Iyer, S.; Thakur, S.; Dixit, M.; Katkam, R.; Agrawal, A.; Kazi, F.
Blockchain and Anomaly Detection Based Monitoring System for
Enforcing Wastewater Reuse. In 2019 10th International Conference on
Computing, Communication and Networking Technologies, ICCCNT
2019; Institute of Electrical and Electronics Engineers Inc., 2019;
DOI: 10.1109/ICCCNT45670.2019.8944586.
(270) Gilmore, A. M.; Chen, L. Improving Aromatic Water-
Contaminant Detection with Machine-Learning Classification and
Regression for Simultaneous Absorbance-Transmission Excitation
Emission Matrix (A-TEEM) Spectroscopy; SPIE-Intl Soc. Optical Eng.
2020, 11233, 55.
(271) Post, C.; Bru
̈
lisauer, S.; Waldschläger, K.; Hug, W.; Gru
̈
neis, L.;
Heyden, N.; Schmor, S.; Förderer, A.; Reid, R.; Reid, M.; Bhartia, R.;
Nguyen, Q.; Schu
̈
ttrumpf, H.; Amann, F. Application of Laser-Induced,
Deep Uv Raman Spectroscopy and Artificial Intelligence in Real-Time
Environmental Monitoring
Solutions and First Results. Sensors 2021,
21 (11), 3911.
(272) Kukula, K.; Farmer, D.; Duran, J.; Majid, N.; Chatterley, C.;
Jessing,J.;Li,Y.RapidDetectionofBacteriaUsingRaman
Spectroscopy and Deep Learning. In 2021 IEEE 11th Annual Computing
and Communication Workshop and Conference, CCWC 2021; Institute of
Electrical and Electronics Engineers Inc., 2021; pp 796−799;
DOI: 10.1109/CCWC51732.2021.9375955.
(273) Sun, C.; Tian, Y.; Gao, L.; Niu, Y.; Zhang, T.; Li, H.; Zhang, Y.;
Yue, Z.; Delepine-Gilon, N.; Yu, J. Machine Learning Allows
Calibration Models to Predict Trace Element Concentration in Soils
with Generalized LIBS Spectra. Sci. Rep. 2019, 9 (1), 1−18.
(274) Asheri Arnon, T.; Ezra, S.; Fishbain, B. Water Characterization
and Early Contamination Detection in Highly Varying Stochastic
Background Water, Based on Machine Learning Methodology for
Processing Real-Time UV-Spectrophotometry. Water Res. 2019, 155,
333−342.
(275) Hall, J.; Zaffiro, A. D.; Marx, R. B.; Kefauver, P. C.; Radha
Krishnan, E.; Haught, R. C.; Herrmann, J. G. On-Line Water Quality
Parameters as Indicators of Distribution System Contamination. J. -
Am. Water Works Assoc. 2007, 99 (1), 66−77.
ACS ES&T Engineering pubs.acs.org/estengg Review
https://doi.org/10.1021/acsestengg.1c00258
ACS EST Engg. XXXX, XXX, XXX−XXX
R

(276) Torregrossa, D.; Hansen, J.; Hernández-Sancho, F.;
Cornelissen, A.; Schutz, G.; Leopold, U. A Data-Driven Methodology
to Support Pump Performance Analysis and Energy Efficiency
Optimization in Waste Water Treatment Plants. Appl. Energy 2017,
208, 1430−1440.
(277) Deveughe
̀
le, S.; Do-Quang, Z. Neural Networks: An Efficient
Approach to Predict on-Line the Optimal Coagulant Dose. Water Sci.
Technol.: Water Supply 2004, 4 (5−6), 87−94.
(278) Ruan, J.; Zhang, C.; Li, Y.; Li, P.; Yang, Z.; Chen, X.; Huang, M.;
Zhang, T. Improving the Efficiency of Dissolved Oxygen Control Using
an On-Line Control System Based on a Genetic Algorithm Evolving
FWNN Software Sensor. J. Environ. Manage. 2017, 187, 550−559.
ACS ES&T Engineering pubs.acs.org/estengg Review
https://doi.org/10.1021/acsestengg.1c00258
ACS EST Engg. XXXX, XXX, XXX−XXX
S

What is it and what are considerations for
its implementation in California
By Arturo A. Keller, Ph.D.
Professor, Bren School UCSB

Start
Start


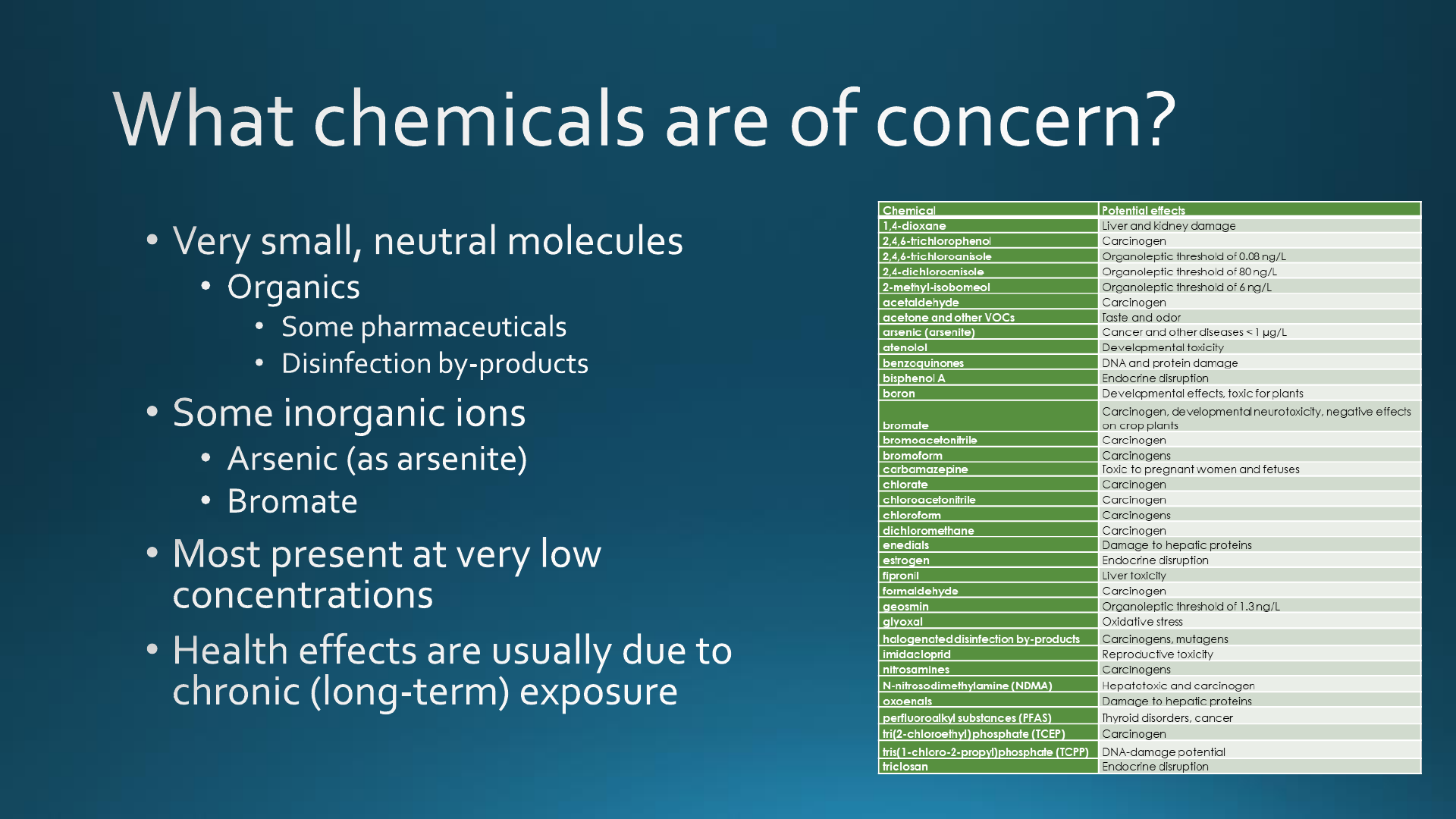

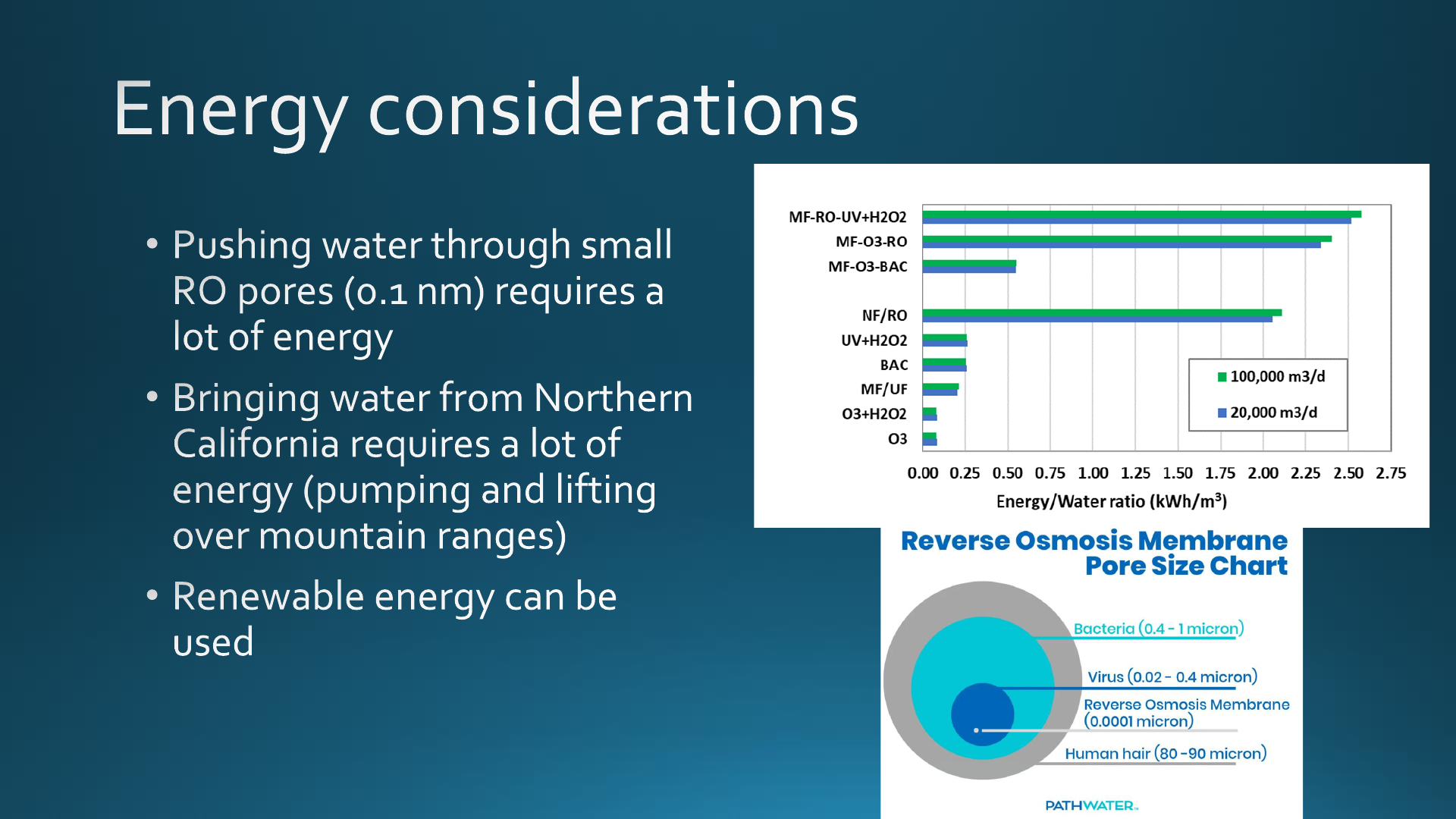



• Establish on-line and real time water quality monitoring systems
• Years away from commercial systems that have very high reliability
• Redundancy in treatment processes to ensure high level of removal
• Will require higher level of investment
• Emergency storage and plans for dealing with off-spec water
• Large enough storage (weeks) near treatment plant
• Where would off-spec water be discharged?
• Use renewable energy
• Consider creative times to treat (peak solar production)
• Assess implementation of DPR very, very carefully
• Conserve, conserve, conserve….!

